Research Articles
From Principles to Practice: A Practical Guide to Implementing the Belmont Report in IRB Protocols
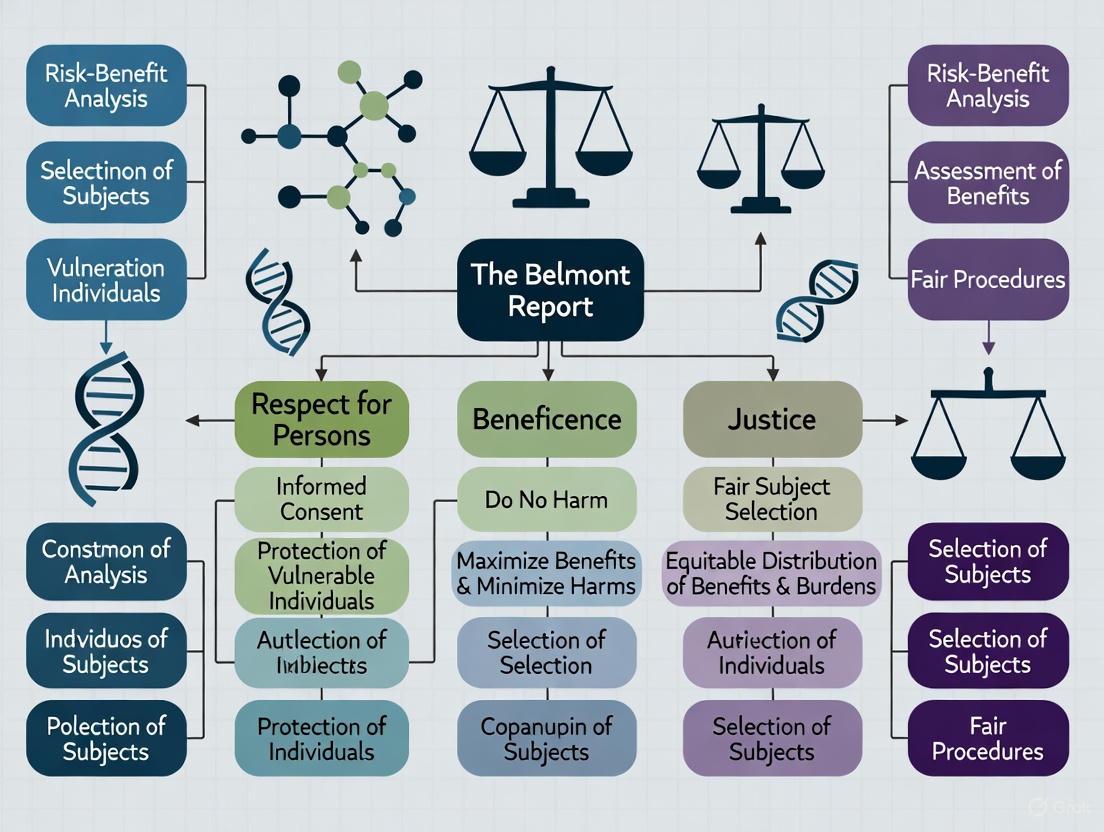
This article provides researchers, scientists, and drug development professionals with a comprehensive framework for applying the Belmont Report's ethical principles—Respect for Persons, Beneficence, and Justice—to Institutional Review Board (IRB) protocols.
Building an Ethical Framework for Human Subjects Research: Principles, Applications, and 2025 Challenges
This article provides a comprehensive guide to ethical frameworks in human subjects research for scientists, researchers, and drug development professionals.
The National Research Act and Belmont Report: A 50-Year Legacy of Ethical Research and Modern Challenges
This article provides a comprehensive analysis of the National Research Act of 1974 and the ensuing Belmont Report, foundational pillars of modern research ethics.
The Common Rule and Belmont Report: A Comprehensive Guide for Research Professionals
This article provides researchers, scientists, and drug development professionals with a complete guide to the U.S.
The Belmont Report in Practice: A Guide to Ethical Principles for Research and Drug Development Professionals
This article provides a comprehensive guide to the three core ethical principles of the Belmont Report—Respect for Persons, Beneficence, and Justice—and their critical application in contemporary biomedical and clinical research.
Ethical Research with Vulnerable Populations: Balancing Protection, Participation, and Progress
This article provides a comprehensive guide for researchers and drug development professionals on the ethical engagement of vulnerable populations in clinical research.
Beyond the Signature: Applying Belmont's Autonomy and Informed Consent in Modern Clinical Research
This article provides a comprehensive analysis of the principle of Respect for Persons and its practical application through informed consent, as defined by the foundational Belmont Report.
From Tuskegee to Belmont: How a Research Scandal Forged Modern Bioethics and Transformed Clinical Practice
This article examines the profound influence of the U.S.
Belmont Report vs Declaration of Helsinki: A Researcher's Guide to Ethical Frameworks
This article provides a comparative analysis for researchers and drug development professionals on the two pillars of research ethics: the US-centric Belmont Report and the globally-oriented Declaration of Helsinki.
Belmont Report vs Nuremberg Code: A Researcher's Guide to Evolving Ethics in Clinical Trials
This article provides a comprehensive comparative analysis of the Nuremberg Code and the Belmont Report, two foundational documents in research ethics.