The Belmont Report in Practice: A Guide to Ethical Principles for Research and Drug Development Professionals
This article provides a comprehensive guide to the three core ethical principles of the Belmont Report—Respect for Persons, Beneficence, and Justice—and their critical application in contemporary biomedical and clinical research.
The Belmont Report in Practice: A Guide to Ethical Principles for Research and Drug Development Professionals
Abstract
This article provides a comprehensive guide to the three core ethical principles of the Belmont Report—Respect for Persons, Beneficence, and Justice—and their critical application in contemporary biomedical and clinical research. Tailored for researchers, scientists, and drug development professionals, it explores the historical foundation of these principles, details their methodological implementation in study design and informed consent, addresses common challenges in balancing ethical obligations, and validates their enduring impact on modern regulations and gene therapy trials. The content is structured to equip professionals with a practical framework for navigating complex ethical dilemmas throughout the research lifecycle.
The Bedrock of Research Ethics: Understanding the Belmont Report's History and Core Principles
The Belmont Report, officially titled "Ethical Principles and Guidelines for the Protection of Human Subjects of Research," stands as a cornerstone document governing ethical conduct in research involving human subjects. Published in 1979 by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, this report emerged from a direct congressional mandate within the National Research Act of 1974 [1] [2]. This legislation was enacted largely in response to growing public outcry over ethical violations in research, most notably the Tuskegee Study, which revealed profound deficiencies in the protection of research participants [2]. The resulting ethical framework provides the foundational principles that continue to guide researchers, scientists, and drug development professionals in the design, review, and conduct of clinical research, ensuring that scientific advancement does not come at the cost of human rights or dignity.
Historical Backdrop: Pathway to Regulation
Pre-Belmont Ethical Codes and Their Limitations
Before the Belmont Report, several international codes attempted to establish ethical standards for human subjects research, but each had significant limitations. The Nuremberg Code, decreed in 1947 during the Doctors' Trial, established the absolute necessity of voluntary consent from competent human subjects free from coercion [3]. While it contained elements related to beneficence and justice, its primary focus was on autonomy, and it was drafted in reference to prisoners in concentration camps, limiting its broader applicability [3]. Subsequently, the Declaration of Helsinki, first adopted in 1964 by the World Medical Association (WMA), placed greater emphasis on the ethical principle of Beneficence and entrusted research ethics committees with approving research protocols [3]. A critical limitation of both documents was their inadequate consideration of protecting socially vulnerable groups such as children or adults with diminished decision-making capacity [3].
The Catalysts for U.S. Action and the National Research Act
In the United States, the Department of Health, Education, and Welfare (DHEW), now the Department of Health and Human Services (HHS), began developing guidance and regulations to address the participation of vulnerable populations in research [3]. However, the pivotal moment came with public disclosure of the Tuskegee Syphilis Study, in which African American men with syphilis were left untreated for decades without their knowledge to study the natural progression of the disease [2]. The ensuing scandal created immense political pressure, leading Congress to pass the National Research Act of 1974 [2]. This Act created the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research and charged it with identifying comprehensive ethical principles for protecting human research subjects [3] [2]. The Commission, meeting at the Belmont Conference Center, developed the report that would carry its name.
Chronology of Key Events
Table 1: Timeline of Key Events Leading to the Belmont Report
| Year | Event | Significance |
|---|---|---|
| 1947 | Nuremberg Code | Established voluntary consent as essential, but context-limited [3]. |
| 1964 | 1st Declaration of Helsinki | Distinguished therapeutic/non-therapeutic research; emphasized beneficence [3]. |
| 1974 | National Research Act | Congressional response to ethical violations; created National Commission [2]. |
| 1979 | Belmont Report Published | Articulated three core ethical principles for human subjects research [1]. |
| Late 1970s-Early 1980s | Common Rule (45 CFR 46) | HHS codified regulations based on Belmont principles [1]. |
Figure 1: The Regulatory Pathway from Ethical Violations to Federal Rulemaking
Core Ethical Principles of the Belmont Report
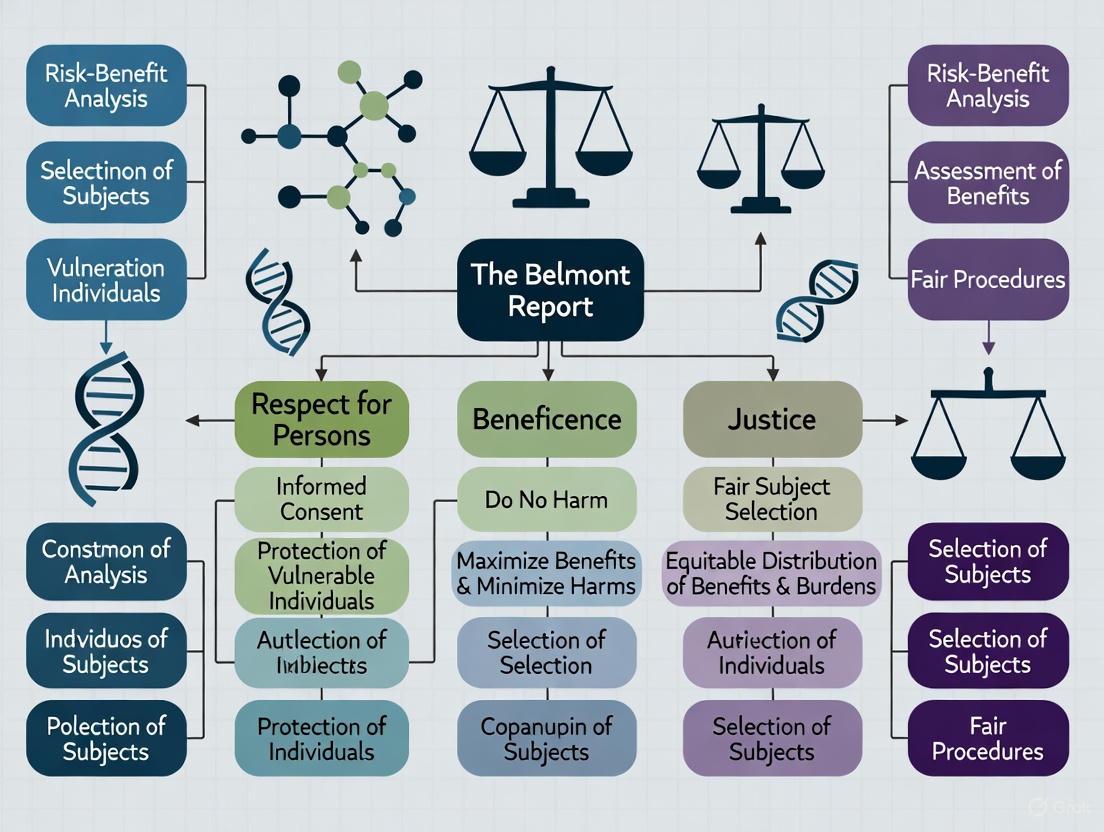
The Belmont Report established three fundamental ethical principles that form the bedrock for the protection of human research subjects: Respect for Persons, Beneficence, and Justice [1] [2]. These principles provide the moral foundation from which specific regulations and procedures are derived.
Respect for Persons
The principle of Respect for Persons incorporates two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection [1]. This principle acknowledges the right of self-determination for individuals capable of deliberating about their personal goals and acting under that direction. Simultaneously, it recognizes that not all individuals are capable of full self-determination due to illness, mental disability, or circumstances that severely restrict liberty [1].
The application of this principle leads to the requirement of informed consent in research. To respect autonomy, investigators must ensure subjects enter research voluntarily with adequate information and comprehension, and that they have the opportunity to ask questions and withdraw from the research at any time without penalty [1]. For populations with diminished autonomy—such as children, prisoners, or individuals with cognitive impairments—additional safeguards must be implemented, which may include exclusion from certain types of research or requiring permission from legally authorized representatives [1].
Beneficence
The principle of Beneficence extends beyond simply refraining from harm to encompass the ethical obligation to maximize possible benefits and minimize possible harms [1] [2]. This principle affirms that persons are treated in an ethical manner not only by respecting their decisions and protecting them from harm, but also by making active efforts to secure their well-being [1].
In practice, beneficence requires a systematic assessment of risks and benefits associated with the research [1] [2]. Investigators and Institutional Review Boards (IRBs) must carefully analyze the nature and scope of risks, including physical, psychological, social, and economic harms, and weigh them against the anticipated benefits to the individual subject and/or the broader society [1]. The Belmont Report cautions that the presence of any research risk necessitates the existence of potential benefits, either to the subject or to society at large [1].
Justice
The principle of Justice requires the fair distribution of the burdens and benefits of research [2]. This principle addresses the ethical concern that no single group in society should disproportionately bear the risks of participation in research while another group reaps the benefits [1] [2].
The application of justice in subject selection requires investigators to examine whether they are systematically selecting subjects simply because of their easy availability, compromised position, or due to social, racial, sexual, or cultural biases [1]. Historically vulnerable populations, such as prisoners, institutionalized individuals, and economically disadvantaged persons, should not be targeted for risky research simply because they are accessible, nor should they be excluded from potentially beneficial research without sound scientific justification [1]. Inclusion and exclusion criteria should be based on those factors that most effectively and soundly address the research problem, not on convenience or social bias [1].
Table 2: The Three Ethical Principles and Their Practical Applications
| Ethical Principle | Core Meaning | Practical Application in Research |
|---|---|---|
| Respect for Persons | Recognition of personal autonomy and protection for those with diminished autonomy. | Informed consent process; additional protections for vulnerable populations. |
| Beneficence | Obligation to maximize benefits and minimize harms. | Systematic assessment of risks and benefits; favorable risk-benefit ratio. |
| Justice | Fairness in distribution of research burdens and benefits. | Equitable selection of research subjects; avoidance of exploiting vulnerable populations. |
The Belmont Report's Enduring Impact on Research Regulation
Influence on Federal Regulations and Institutional Review
The Belmont Report directly influenced the development and revision of federal regulations for human subjects protection, most notably the Common Rule (45 CFR 46), which established uniform policies across multiple federal agencies [1] [3]. The Report also provided the analytical framework for the Institutional Review Board (IRB) system, giving IRBs a structured method to determine whether the risks to research subjects are justified by the potential benefits [1]. According to this method, IRBs systematically gather and assess information about all aspects of proposed research, consider alternatives, and communicate their findings to investigators in a factual and precise manner [1].
Modern Applications in Drug Development and Clinical Research
The principles of the Belmont Report continue to guide contemporary research oversight and practice. Recent FDA guidance documents demonstrate how these ethical foundations evolve to address new challenges while maintaining their core commitments:
- Informed Consent and Respect for Persons: The FDA's 2025 draft guidance, "Evaluation of Sex-Specific and Gender-Specific Data in Medical Device Clinical Studies," encourages science-driven consideration of sex and gender to ensure medical devices are safe and effective for all potential users, reflecting the principle of justice and respect for personhood [4].
- Beneficence in Risk Assessment: The FDA's 2025 final guidance, "Action Levels for Lead in Processed Food Intended for Babies and Young Children," directly applies beneficence by establishing limits to reduce dietary exposure to contaminants in vulnerable populations [4].
- Justice in Subject Selection: Recent FDA initiatives, including the draft guidance "Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies," directly address the principle of justice by working to ensure clinical trial populations better represent the patients who will ultimately use medical products [4].
Ethical Framework for Emerging Research Technologies
The Belmont principles also provide guidance for navigating ethical challenges in cutting-edge research areas. For instance, the FDA and NIH have issued updated recommendations on human cells, tissues, and cellular and tissue-based products (HCT/Ps) that eliminate previous screening questions specific to men who have sex with men (MSM) and instead recommend assessing donor eligibility using the same individual risk-based questions for every donor, regardless of sex or gender [4]. This policy shift reflects an evolving application of the justice principle, reducing discrimination while maintaining safety.
Similarly, new policies regarding the security of human biospecimens and associated data, including restrictions on distribution to countries of concern, reflect contemporary applications of the beneficence principle by seeking to protect research participants and national interests in an increasingly complex global research environment [5].
The Researcher's Toolkit: Implementing Belmont Principles
Table 3: Essential Tools for Implementing Ethical Research Practices
| Tool/Component | Function in Ethical Research | Belmont Principle Addressed |
|---|---|---|
| Informed Consent Document | Provides comprehensive information about the study in understandable language; ensures voluntary participation. | Respect for Persons |
| Institutional Review Board (IRB) Protocol | Detailed research plan submitted for ethical review, including rationale, methodology, and participant protections. | Beneficence, Justice |
| Risk-Benefit Assessment Matrix | Systematic tool for identifying, quantifying, and weighing potential harms against anticipated benefits. | Beneficence |
| Participant Recruitment Materials | Advertisements, brochures, and scripts designed to enroll subjects without coercion or undue influence. | Respect for Persons, Justice |
| Data Safety Monitoring Plan | Procedures for ongoing review of accumulated data to ensure participant safety and study validity. | Beneficence |
| Vulnerable Population Safeguards | Additional protections (e.g., assent procedures, legal representatives) for those with diminished autonomy. | Respect for Persons, Justice |
| Diversity and Inclusion Plan | Strategy to ensure equitable enrollment across demographic groups relevant to the research question. | Justice |
The journey From the National Research Act to the Belmont Report represents a pivotal transformation in the American research landscape, establishing an enduring ethical framework that continues to guide scientific inquiry. The three principles of Respect for Persons, Beneficence, and Justice provide more than historical guidance; they form a living analytical framework that adapts to new scientific challenges while maintaining fundamental protections for human dignity. For today's researchers, scientists, and drug development professionals, understanding this historical context and the profound ethical principles embedded in the Belmont Report remains essential for conducting scientifically valid and ethically sound research that advances knowledge while honoring our shared commitment to human rights.
This whitepaper provides an in-depth technical analysis of the three foundational ethical principles—Respect for Persons, Beneficence, and Justice—as defined by the Belmont Report. Framed within the context of human subjects research regulation, this guide examines the historical imperatives, detailed applications, and ongoing relevance of these principles for contemporary researchers, scientists, and drug development professionals. The document synthesizes original regulatory text with modern interpretations to offer a comprehensive ethical framework supported by structured data tables, procedural workflows, and practical implementation tools designed to ensure rigorous protection of human research participants.
The Belmont Report was designed to identify basic ethical principles and develop guidelines that should govern research involving human subjects, thereby preventing such abuses [1]. It serves as the primary ethical foundation for the Federal Policy for the Protection of Human Subjects (the "Common Rule") and is cited by institutions like the University of Wisconsin-Madison as the basis for protecting the rights and welfare of research subjects [1]. The report established three core principles—Respect for Persons, Beneficence, and Justice—which form the essential pillars for the ethical conduct of research [6].
The Ethical Principles: Conceptual Foundations
Respect for Persons
The principle of Respect for Persons incorporates two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection [1]. This principle acknowledges the personal dignity and liberty of individuals, requiring that researchers acknowledge autonomy and protect those with reduced autonomy, such as children, individuals with cognitive disabilities, or prisoners [6]. The moral requirements here involve acknowledging autonomy and providing protection for the vulnerable.
Beneficence
The principle of Beneficence extends beyond merely refraining from harm to encompassing the obligation to maximize possible benefits and minimize potential harms [1] [6]. This principle is often expressed in a two-part formulation: (1) do not harm, and (2) maximize possible benefits while minimizing possible harms [6]. In research practice, this requires a systematic assessment of risks and benefits to ensure the well-being of research subjects is secured.
Justice
The principle of Justice requires the fair distribution of both the burdens and benefits of research [1]. It addresses the ethical obligation to ensure that exploitative research is avoided and that the selection of research subjects is scrutinized to determine whether some classes are being systematically selected simply because of their easy availability, compromised position, or social status rather than for reasons directly related to the research problem [6]. This principle guards against the exploitation of vulnerable populations.
Table 1: Core Ethical Principles of the Belmont Report
| Ethical Principle | Core Concept | Moral Imperative | Vulnerable Populations Considerations |
|---|---|---|---|
| Respect for Persons | Individual autonomy and protection of those with diminished autonomy | Recognize voluntary, informed choice; provide special protection for diminished autonomy | Children, prisoners, individuals with cognitive or psychological impairments [6] |
| Beneficence | Obligation to maximize benefits and minimize harms | Do not harm; maximize possible benefits and minimize possible risks | Requires heightened assessment for all participants, with extra protections for vulnerable groups [6] |
| Justice | Fairness in distribution of research burdens and benefits | Ensure equitable selection of subjects; avoid exploitation of vulnerable populations | Ethnic and racial minorities, economically disadvantaged, the institutionalized [6] |
Practical Application: Translating Principles to Practice
The Belmont Report outlines how its ethical principles should be applied in research practice through three key areas: Informed Consent, Assessment of Risks and Benefits, and Selection of Subjects [3].
Application: Informed Consent
The application of Informed Consent flows directly from the principle of Respect for Persons. To respect individual autonomy, researchers must ensure subjects enter research voluntarily and with adequate information [1].
Table 2: Essential Elements of Valid Informed Consent
| Element Category | Specific Component | Technical Requirement | Ethical Principle Served |
|---|---|---|---|
| Information | Research procedures & purposes | Disclosed in comprehensible language | Respect for Persons |
| Risks & anticipated benefits | Clearly explained, including likelihood | Beneficence | |
| Alternative procedures | Disclosed (where therapy is involved) | Respect for Persons, Beneficence | |
| Comprehension | Subject understanding | Information presented in understandable manner | Respect for Persons |
| Adaptation to subject capacity | Tailored to subject's comprehension level | Respect for Persons | |
| Voluntariness | Absence of coercion | Free power of choice without constraint | Respect for Persons, Justice |
| Right to withdraw | Statement offering subject opportunity to ask questions and withdraw at any time | Respect for Persons |
Application: Assessment of Risks and Benefits
The systematic Assessment of Risks and Benefits is the practical application of the principle of Beneficence. This requires a careful, systematic documentation and analysis of all potential risks and benefits, both to individual subjects and to society at large [1]. Researchers and IRBs must distinguish between the scientific validity of a research design and the assessment of risks and benefits, as a poorly designed study inherently exposes subjects to risk without the potential benefit of valuable knowledge [1]. The assessment process must be rigorous and factual, considering alternative ways to obtain the benefits sought.
Application: Selection of Subjects
The Selection of Subjects is the primary application of the principle of Justice. It requires scrutiny to ensure that the burdens of research are not disproportionately borne by any particular group, especially those vulnerable to exploitation, while the benefits of research are distributed fairly [6]. Research should not systematically select certain groups (e.g., participants receiving public financial assistance, specific ethnic and racial minorities, or the institutionalized) simply because of their easy availability or compromised position [1]. The inclusion and exclusion criteria must be based on factors that most effectively and soundly address the research problem, not social convenience [1].
Diagram 1: Belmont Report Principles to Application Mapping
Methodological Implementation: The Researcher's Toolkit
Institutional Review Board (IRB) Protocol
The Institutional Review Board (IRB) serves as the primary mechanism for enforcing the ethical principles of the Belmont Report in research practice [6]. Researchers must submit a complete research proposal to the IRB, including specific data collection instruments, research advertisements, and informed consent documentation before initiating any research activities [6]. The IRB review process involves:
- Full Committee Review: Required for research involving more than minimal risk
- Expedited Review: For certain categories of research involving no more than minimal risk
- Exempt Determination: For specific categories of research that qualify for exemption
The IRB composition intentionally includes experts across various disciplines, including ethicists, social workers, physicians, nurses, scientific researchers, and legal experts to provide comprehensive ethical oversight [6].
Research Reagent Solutions: Ethical Implementation Framework
Table 3: Essential Methodological Components for Ethical Research Implementation
| Component | Function | Implementation Guideline |
|---|---|---|
| Comprehensive Protocol | Details all research procedures and methodologies for IRB review | Must include specific data collection instruments, advertisements, and consent documentation [6] |
| Informed Consent Documentation | Ensures voluntary participation with adequate information | Must include research procedures, purposes, risks, benefits, alternatives, and right to withdraw [1] [6] |
| Vulnerable Population Safeguards | Provides additional protections for vulnerable groups | Requires special consent procedures, assent for children, and additional oversight [6] |
| Risk-Benefit Assessment Framework | Systematically analyzes potential harms and benefits | Must document all potential risks (physical, psychological, social, economic) and benefits [1] |
| Data Confidentiality Protocols | Protects participant privacy and confidentiality | Includes procedures for data anonymization, secure storage, and confidentiality assurance [6] |
| Subject Selection Justification | Ensures equitable selection of research subjects | Must document that selection is based on scientific requirements, not convenience or vulnerability [1] |
Risk-Benefit Assessment Methodology
The assessment of risks and benefits requires a systematic, analytical approach guided by the following methodological considerations:
- Brutal Data Collection: Gather complete information about all aspects of the research, considering even remote possibilities of harm
- Systematic Consideration of Alternatives: Evaluate whether comparable knowledge could be gained through less-risky methods
- Non-arbitrary Analysis: Ensure the assessment process is rigorous and based on factual evidence rather than intuition
- Ongoing Evaluation: Continue assessment throughout the research lifecycle, not just during initial review
This methodological framework ensures that the analysis of risks and benefits is comprehensive and scientifically valid, fulfilling the ethical obligation of beneficence.
Diagram 2: Ethical Research Protocol Implementation Workflow
Discussion: Contemporary Relevance in Research
The Belmont Report's principles continue to provide the fundamental framework for ethical research conduct, particularly in the evolving landscape of drug development and clinical trials. The principles have been clearly reflected in regulations governing gene therapy clinical trials and policies regarding public review of protocols [3]. For modern researchers and drug development professionals, these principles offer a robust structure for addressing emerging ethical challenges in areas including:
- Precision Medicine: Ensuring equitable access to emerging targeted therapies
- Genetic Research: Protecting privacy and autonomy in the context of complex genetic information
- Global Clinical Trials: Applying justice principles to prevent exploitation in international research settings
- Digital Health Technologies: Assessing risks and benefits in rapidly evolving technological environments
- Vulnerable Population Research: Maintaining rigorous protections while enabling essential research
The three principles provide a comprehensive framework that continues to guide researchers in balancing scientific advancement with rigorous ethical practice, ensuring that the fundamental rights and welfare of human subjects remain protected in an increasingly complex research landscape.
The Belmont Report, formally titled "Ethical Principles and Guidelines for the Protection of Human Subjects of Research," was published in 1979 by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research [3] [1]. This foundational document emerged in response to historical abuses in human subjects research, most notably the Tuskegee Syphilis Study, where uninformed and unaware patients were exposed to disease or subjected to unproven treatments without their consent [6] [7]. The report established three fundamental ethical principles to govern research involving human subjects: respect for persons, beneficence, and justice [8] [9].
The principle of respect for persons serves as the ethical foundation for the treatment of human research participants and incorporates two distinct but related ethical convictions [8] [9]. First, individuals should be treated as autonomous agents capable of making responsible choices and determining their own life course. Second, persons with diminished autonomy are entitled to special protections to safeguard their well-being and rights [1]. This principle divides into two moral requirements: the requirement to acknowledge autonomy through informed consent processes, and the requirement to protect those with diminished autonomy through additional safeguards [8]. Within the framework of the Belmont Report, respect for persons finds its primary application in the process of informed consent, ensuring that individuals voluntarily choose whether to participate in research after receiving comprehensive information presented in an understandable format [9].
Core Components of Respect for Persons
Autonomy in Research Ethics
The concept of autonomy is central to the principle of respect for persons. An autonomous person is "an individual capable of deliberation about personal goals and acting under the direction of such deliberation" [6]. In research practice, respecting autonomy means acknowledging individuals' right to make their own decisions without coercion or undue influence [10]. Researchers must ensure participants have the right to self-determination, including the right to decide whether to participate voluntarily, to ask questions, and to comprehend the questions asked by the researcher [6].
Cultural considerations significantly influence the practical application of autonomy. Western societies typically emphasize individual autonomy, where decision-making revolves around a single person. In contrast, collectivistic cultures often practice collective autonomy, where decision-making is group-oriented, shared, or designated to specific individuals [11]. For example, research involving South Asian immigrants may require a family-centered approach to decision-making, while working with Indigenous groups may necessitate deference to tribal leaders or community gatekeepers [11]. These cultural variations require researchers to adopt culturally sensitive approaches to autonomy that respect diverse decision-making frameworks while maintaining ethical integrity.
Informed Consent Process
Informed consent represents the practical application of respect for persons in research settings [9]. The Belmont Report analyzes the consent process as containing three essential elements: information, comprehension, and voluntariness [9]. Each element must be adequately addressed to ensure meaningful consent rather than mere ritualistic documentation.
Information: Researchers must provide complete details about the research study, including procedures, purposes, risks and anticipated benefits, alternative procedures (where therapy is involved), and a statement offering subjects the opportunity to ask questions and to withdraw at any time [1]. The information must be presented in language understandable to the participant, avoiding technical jargon that might obscure meaning.
Comprehension: Researchers must ensure that potential participants truly understand the information provided. This requires presenting information in a manner commensurate with the participant's comprehension level and ensuring the communication process is effective [9]. For populations with educational disadvantages or cognitive impairments, researchers may need to employ additional strategies to verify understanding.
Voluntariness: Consent must be given voluntarily without coercion or undue influence [9]. Researchers must avoid threats of penalty (whether explicit or implied) for declining participation and must carefully consider whether excessive rewards might compromise voluntary choice [6]. The environment and power dynamics between researcher and participant can significantly impact voluntariness.
Table 1: Essential Elements of Valid Informed Consent
| Element | Key Requirements | Common Pitfalls |
|---|---|---|
| Information Disclosure | Research procedures, purposes, risks, benefits, alternatives, right to withdraw | Use of technical jargon, overwhelming detail, omission of significant risks |
| Comprehension | Information tailored to participant understanding, verification of understanding | Assuming understanding without assessment, ignoring cultural/language barriers |
| Voluntariness | Absence of coercion or undue influence, freedom to refuse without penalty | Power differentials, excessive incentives, implicit pressure from researchers |
Protecting Persons with Diminished Autonomy
The second ethical conviction within respect for persons recognizes that some individuals have diminished autonomy and require additional protections [8]. The extent of protection should be proportionate to the risk of harm and likelihood of benefit, and the judgment about an individual's autonomy should be periodically reevaluated as it may vary in different situations [1]. Certain populations are specifically identified as vulnerable in federal regulations, while others may exhibit nuanced vulnerability depending on contextual factors [11].
Table 2: Populations with Diminished Autonomy and Protective Considerations
| Population | Nature of Vulnerability | Recommended Protections |
|---|---|---|
| Children | By regulatory definition, persons who have not attained legal age for consent [11] | Parental permission and child assent (for children 7+), justification for inclusion based on research purpose [6] [11] |
| Prisoners | Involuntarily confined individuals with compromised ability to refuse [8] [11] | Prisoner representative on IRB, ensure benefits not overly influential, fair selection procedures [8] [11] |
| Individuals with Impaired Decision-Making Capacity | Wide variety of conditions affecting understanding and weighing of information [11] | Periodic check-ins, assessment of consent capacity, surrogate decision-makers, assent when possible [6] [11] |
| Economically/Eductionally Disadvantaged | Potential vulnerability to undue influence due to limited resources [11] | Additional safeguards against coercion, tailored communication approaches, consideration of alternative consent documentation [11] |
| Culturally Vulnerable Groups | Historical exploitation or different decision-making norms [11] | Cultural humility, community consultation, collective consent approaches when appropriate [11] |
Practical Applications and Methodologies
Implementing Ethical Protocols
Translating the principle of respect for persons into actionable research protocols requires systematic approaches at each stage of the research process. The following workflow illustrates the key stages in implementing ethical protocols for respect for persons:
For research involving vulnerable populations with impaired decision-making capacity, specific methodological approaches are necessary:
Capacity Assessment Protocols: Implement brief, validated tools to assess potential participants' understanding of research elements, including purpose, procedures, risks, benefits, and alternatives [11]. Document assessment results and capacity determination.
Surrogate Decision-Maker Engagement: Identify legally authorized representatives according to state hierarchy (typically spouse, adult children, parents, siblings) [12]. Provide complete information to surrogates and ensure they understand their role in representing the participant's best interests and known preferences.
Assent Procedures: Even when formal consent comes from a surrogate, seek affirmative agreement from participants with diminished autonomy to the extent of their capabilities [6]. Use age-appropriate or capacity-appropriate materials and periodically re-evaluate willingness to continue participation.
Cultural Consultation: For research involving cultural subgroups, engage cultural consultants or community advisory boards during study design to ensure appropriate consent approaches [11]. This may involve community-level consent before individual consent in some Indigenous populations.
Assessment Tools and Documentation
Robust assessment and documentation are essential for demonstrating adherence to the principle of respect for persons. Researchers should employ multiple methods to verify understanding and maintain comprehensive records of the consent process:
Teach-Back Method: Ask participants to explain the study in their own words to verify comprehension of key elements including purpose, procedures, risks, benefits, and voluntary nature [6].
Decisional Capacity Assessments: For populations with potential cognitive impairments, use structured assessments such as the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) to evaluate understanding, appreciation, reasoning, and expression of choice [11].
Vulnerability Mitigation Checklists: Develop population-specific checklists to ensure appropriate safeguards are implemented, such as independent witnesses for consent processes, additional educational materials, or community advocate involvement [11].
Cultural Sensitivity Reviews: Have consent materials and processes reviewed by individuals familiar with the cultural context of the participant population to identify potential barriers or unintended cultural offenses [11].
Table 3: Research Ethics Toolkit for Respect for Persons
| Tool Category | Specific Instruments | Application Context |
|---|---|---|
| Comprehension Verification | Teach-back method, Quizzes, Open-ended questioning | All research populations, especially with complex protocols or educational limitations |
| Capacity Assessment | MacCAT-CR, UBACC, Mini-Mental State Examination (MMSE) | Research involving elderly, cognitively impaired, or psychiatric populations |
| Cultural Adaptation | Community advisory boards, Cultural consultants, Translated materials | Research with ethnic minorities, Indigenous populations, or international sites |
| Documentation Alternatives | Verbal consent with witnesses, Electronic consent with multimedia, Short forms | Populations with literacy challenges, undocumented individuals, low-risk studies |
Institutional Review Board Evaluation Criteria
Institutional Review Boards (IRBs) systematically evaluate research protocols to ensure compliance with the principle of respect for persons. Researchers should be prepared to address the following criteria during IRB review [1] [6]:
Consent Process Rigor: The IRB examines the proposed consent process for completeness of information, appropriateness for the participant population, and procedures to ensure comprehension and voluntariness [6]. Boards may require simplified language, visual aids, or independent consent monitors for vulnerable populations.
Vulnerability Assessment: Researchers must justify the inclusion of vulnerable populations and demonstrate appropriate safeguards [6] [11]. The IRB evaluates whether the research could be conducted with less vulnerable populations and whether risks are justified by potential benefits to participants or the vulnerable group.
Cultural Competence: The IRB assesses whether researchers have demonstrated understanding of and appropriate approaches to cultural factors that may impact participant autonomy and decision-making [11]. This includes consideration of collective decision-making norms, language barriers, and historical trauma.
Privacy and Confidentiality: Protocols must include adequate measures to protect participant privacy through data anonymization or confidentiality through secure data handling [6]. The IRB evaluates these measures in relation to the sensitivity of the data collected.
Contemporary Challenges and Evolving Considerations
Nuanced Vulnerabilities in Modern Research
While federal regulations specify certain vulnerable populations, contemporary research recognizes the concept of nuanced vulnerability - groups that may not be listed in regulations but require augmented protections depending on context [11]. These include:
Undocumented Immigrants: Who may fear that signing consent forms could have legal repercussions, requiring alternatives to standard written documentation [11].
Gender-Oppressed Groups: Women in certain cultural contexts whose decision-making may be constrained by familial or religious structures, necessitating consultation with designated family members [11].
Historically Exploited Communities: Such as Native American tribes with historical trauma from previous research exploitation, requiring community engagement and sometimes tribal approval before individual consent [11].
Researchers must practice cultural humility - an ongoing process of self-reflection and self-critique about cultural norms and recognition of power imbalances in researcher-participant relationships [11]. This approach acknowledges that researchers cannot be complete experts on other cultures but must engage in lifelong learning to develop mutually beneficial and respectful research partnerships.
Regulatory Framework and Global Applications
The principles outlined in the Belmont Report continue to influence contemporary regulatory frameworks, including the International Council for Harmonisation's (ICH) Guideline for Good Clinical Practice E6(R3) [7]. The report's enduring relevance nearly five decades after its publication demonstrates the robustness of its ethical framework while requiring adaptation to evolving research contexts [7].
The Common Rule (45 CFR Part 46) codifies the principles of the Belmont Report into federal regulations governing human subjects research in the United States [12] [7]. Additional FDA regulations (21 CFR Parts 50, 56) provide specific requirements for informed consent and IRB operations in clinical investigations [12]. State statutes may provide additional protections, such as California's requirements for minor assent in experimental drug studies [12].
In global research contexts, the principle of respect for persons requires careful navigation of diverse cultural norms while maintaining ethical essentials. This may involve implementing multi-level consent processes (community, familial, and individual) in collectivist cultures while still ensuring individual participants understand they may decline participation without collective repercussions [11]. The fundamental requirements of understanding, voluntariness, and protection of vulnerable individuals remain constant across cultural contexts, though their implementation may vary appropriately.
The principle of respect for persons, as articulated in the Belmont Report, remains a cornerstone of ethical research involving human subjects nearly five decades after its publication [7]. Its two-fold requirement - to acknowledge autonomy through informed consent processes and to protect those with diminished autonomy through additional safeguards - provides a robust framework for ethical decision-making in research [8] [1]. As research methodologies and participant populations evolve, the enduring relevance of this principle requires researchers to continually adapt its application to new contexts while maintaining fidelity to its ethical foundations. By embracing both the letter and spirit of respect for persons through culturally humble practices, rigorous consent processes, and appropriate protections for vulnerable populations, researchers honor the dignity and worth of every individual who contributes to the advancement of knowledge through research participation.
Within the foundational ethical framework of the Belmont Report, the principle of beneficence imposes a dual obligation upon researchers: to "do no harm" and to "maximize possible benefits and minimize possible harms" [1]. This principle extends beyond merely refraining from inflicting injury; it requires an active commitment to securing the well-being of research participants and society at large [1]. For researchers, scientists, and drug development professionals, navigating this dual obligation is a complex, continuous process integral to every stage of experimental design and execution. Beneficence ensures that the research enterprise is not only scientifically valid but also ethically defensible, demanding a rigorous and systematic assessment of risks and benefits long before the first participant is enrolled [13]. This guide provides a technical roadmap for applying this critical principle, featuring structured frameworks for risk-benefit analysis, practical methodological checklists, and visualization of ethical decision-making pathways to uphold the highest standards of research integrity.
The Ethical Framework of Beneficence
Historical and Regulatory Context
The Belmont Report, published in 1979, emerged as a direct response to historical ethical failures in research, most notably the Tuskegee Syphilis Study [7]. It established beneficence as one of three core principles—alongside Respect for Persons and Justice—that would form the bedrock for modern regulations protecting human subjects [1] [3]. This principle was codified into U.S. federal law through the Common Rule (45 CFR 46), which governs nearly all federally funded human subjects research in the United States [14]. The Belmont Report's enduring influence is further evidenced by its alignment with contemporary guidelines, such as the International Council for Harmonisation's (ICH) Guideline for Good Clinical Practice E6(R3), which is followed by clinical researchers worldwide [7].
The Dual Rules of Beneficence
The principle of beneficence is operationalized through two complementary moral rules [1]:
- Do not harm: This rule, often associated with the Hippocratic tradition, prohibits the intentional infliction of injury or harm on research subjects.
- Maximize possible benefits and minimize possible harms: This positive obligation requires researchers to proactively enhance the value of the research while systematically reducing and managing its inherent risks.
In practice, these rules necessitate that any risks to participants must be justified by the anticipated benefits, either to the individual subject or to society through the advancement of knowledge [1] [13]. This favorable risk-benefit ratio is a cornerstone of ethical research and a primary focus for Institutional Review Board (IRB) review [13].
Operationalizing Beneficence: A Methodological Framework
Systematic Risk-Benefit Assessment
A rigorous, non-arbitrary assessment of risks and benefits is the primary methodology for fulfilling the obligation of beneficence. The Belmont Report itself outlines a method for IRBs to use in this determination, which researchers should preemptively apply during study design [1]. The process involves gathering and assessing all available information on the research components and systematically considering alternatives.
Table: Taxonomy of Research Risks and Benefits
| Category | Types & Examples | Assessment Considerations |
|---|---|---|
| Risks | Physical: Pain, infection, side effects from an investigational drug [13].Psychological: Stress, anxiety, emotional distress [13].Social: Stigma, breach of confidentiality, impact on employability [13].Economic: Financial costs of participation, travel time, lost wages [13]. | - Probability of occurrence.- Severity (trivial to serious).- Duration (transient to long-term).- Reversibility. |
| Benefits | Direct to Subject: Access to a potentially therapeutic intervention, improved health monitoring, financial compensation [14].Societal: Acquisition of generalizable knowledge to improve future health, development of new diagnostic tools or treatments [1] [14]. | - Likelihood of realization.- Magnitude.- Specificity to the participant population. |
The Risk-Benefit Workflow
The following diagram illustrates the systematic, iterative workflow required for a ethically sound risk-benefit assessment, from initial identification through to final IRB review and ongoing monitoring.
The Scientist's Toolkit: Essential Materials for Ethical Research
Upholding beneficence requires more than ethical intent; it relies on specific tools and documents that structure and document the protection of participant welfare.
Table: Essential Research Reagent Solutions for Ethical Practice
| Tool / Reagent | Function in Upholding Beneficence |
|---|---|
| Protocol with Stopping Rules | A pre-defined, detailed research plan that includes clear stopping rules and safety thresholds to prevent undue harm. |
| Data Safety Monitoring Board (DSMB) | An independent committee of experts that reviews accumulated data from a clinical trial at intervals to ensure participant safety and study validity. |
| Informed Consent Document | The primary tool for communicating the research's foreseeable risks, potential benefits, and alternatives to potential participants [1] [13]. |
| Adverse Event (AE) Reporting System | A standardized system for capturing, documenting, and reporting any untoward medical occurrences in a research participant. |
| Institutional Review Board (IRB) Application | The formal submission that justifies the research to an independent review panel, demonstrating a favorable risk-benefit ratio and ethical design [13]. |
Navigating Complexities and Conflicts
Balancing Beneficence with Other Ethical Principles
The ethical principles of the Belmont Report do not exist in isolation and can sometimes conflict. A classic example occurs in research involving children. The principle of Respect for Persons requires honoring a child's dissent, while Beneficence might argue for a potential direct therapeutic benefit from participation, and Justice supports including children in research to gain knowledge relevant to their health conditions [14]. Regulatory frameworks provide guidance for resolving these tensions; for instance, in greater-than-minimal risk research offering direct benefit to the child, an IRB may allow a parent's permission to override a child's dissent in the interest of the child's well-being (favoring Beneficence) [14].
The Challenge of Uncertainty
A fundamental challenge in applying beneficence is the inherent uncertainty about the degree of risks and benefits in any clinical research study [13]. By definition, research seeks to answer questions for which the answers are not yet known. This underscores the necessity of the systematic assessment process, ongoing monitoring, and the communication of this uncertainty to participants during the informed consent process [13].
The framework of beneficence remains critically relevant in addressing modern research challenges. The rise of Dual Use Research of Concern (DURC)—research with a legitimate scientific purpose that could be misapplied to pose a significant threat—explicitly engages the principle of beneficence by forcing a consideration of potential harms to public health and security on a broad scale [15]. Similarly, recent federal initiatives like the U.S. Gold Standard Science executive order, which emphasizes reproducibility, transparency, and communication of error, are rooted in the beneficent goal of ensuring reliable and credible science [16].
In conclusion, for the research professional, beneficence is not a passive ideal but an active, methodological practice. It requires a vigilant, systematic, and documented commitment to the dual obligation of avoiding harm and optimizing benefits throughout the research lifecycle. By integrating the structured assessments, workflows, and tools outlined in this guide, researchers can ensure their work not only advances scientific knowledge but also faithfully honors the trust placed in them by participants and society.
The Belmont Report, published in 1978, establishes a foundational ethical framework for research involving human subjects, built upon three core principles: respect for persons, beneficence, and justice [7] [1]. This guide focuses on the principle of justice, which pertains to the fair distribution of the benefits and burdens of research [17]. In practice, justice requires that the selection of research subjects must be scrutinized to avoid systematic selection based on easy availability, compromised position, or societal manipulability [18]. Instead, subject selection should be based on factors directly related to the research problem, ensuring that no specific group (whether defined by gender, race, ethnicity, or socioeconomic status) is unfairly burdened with the risks of research or denied its benefits [18] [1]. For researchers and drug development professionals, operationalizing this principle is a critical component of ethical study design and regulatory compliance.
The Conceptual Framework of Justice
Historical Context and Ethical Foundations
The emphasis on justice in research ethics arose from a history of exploitative practices where the burdens of research fell disproportionately on vulnerable populations. The Belmont Report itself cites historical examples, including:
- Poor ward patients in the 19th and early 20th centuries who bore the burdens of research while benefits flowed primarily to private patients.
- Unwilling prisoners in Nazi concentration camps who were exploited as research subjects.
- Disadvantaged rural Black men in the Tuskegee syphilis study, who were deprived of effective treatment long after it became available [18].
These injustices highlighted the need for a systematic application of justice to ensure the fair selection of subjects and equitable distribution of research risks and benefits.
Conceptions of Justice in Research
The Belmont Report primarily embodies a conception of distributive justice—the fair allocation of society's benefits and burdens [18]. However, other conceptions are also relevant:
- Distributive Justice: Pertains to the fair allocation of the benefits and burdens of research. It requires that no one group should receive disproportionate benefits or bear disproportionate burdens [18].
- Procedural Justice: Applies to matters where a fair result depends on adherence to well-ordered procedures, such as the requirements of due process in informed consent and Institutional Review Board (IRB) review [18].
- Compensatory Justice: Goes beyond fairness in distribution to remedy or redress past wrongs, such as the monetary payments made to survivors of the Tuskegee syphilis study [18].
A key requirement of justice in research is a "fitting" match: the population from which research subjects are drawn should reflect the population to be served by the results of the research [18].
Regulatory and Guidance Framework
The Common Rule and International Harmonization
The ethical principles of the Belmont Report are codified in the U.S. Federal Policy for the Protection of Human Subjects, known as the Common Rule (45 CFR 46) [17] [7]. The Common Rule mandates that research conducted or funded by federal agencies must adhere to requirements for informed consent, IRB review, and equitable subject selection [17].
For global drug development, sponsors must navigate both the International Council for Harmonisation (ICH) guidelines and U.S.-specific regulations [19]. While both frameworks are rooted in the same ethical principles, critical distinctions exist:
Table: Comparison of ICH Guidelines and U.S. Common Rule
| Feature | ICH Guidelines | U.S. Common Rule |
|---|---|---|
| Primary Focus | Clinical trials for pharmaceuticals [19] | Broad range of human subjects research [19] |
| Legal Status | Recommendations adopted into national laws [19] | Legally binding regulation in the U.S. [19] |
| IRB Composition | -- | Emphasizes diversity (race, gender, culture, profession) [19] |
| Documentation | -- | Often requires more extensive documentation and financial disclosures [19] |
Contemporary FDA Guidance on Representativeness
Recent FDA draft guidances emphasize the importance of representativeness in clinical trials, particularly for multiregional clinical trials (MRCTs). Key considerations include:
- U.S. Participant Representation: Data submitted for FDA marketing approval should include results from a substantial number of U.S. participants, even if subjects in other regions share similar characteristics [19].
- Standard of Care Evaluation: Differences in standard-of-care treatment at foreign sites compared to the U.S. must be evaluated [19].
- Beyond Demographics: Representativeness should consider Social Determinants of Health (e.g., socioeconomic status, healthcare access, environmental factors) that significantly impact health outcomes and treatment responses, moving beyond mere racial and ethnic categories [19].
Practical Application: Methodologies for Ensuring Justice
Designing Equitable Inclusion and Exclusion Criteria
The first step in ensuring justice is the formulation of study eligibility criteria that are scientifically justified and non-discriminatory.
- Protocol Development Tool: The following diagram outlines a decision pathway for developing equitable study criteria.
Strategies for Recruiting a Representative Sample
Achieving a representative sample requires proactive, structured strategies. The following experimental protocol provides a methodological framework for recruitment.
Table: Protocol for Recruiting a Representative Sample
| Protocol Step | Detailed Methodology | Tools & Considerations |
|---|---|---|
| 1. Population Mapping | Analyze disease epidemiology and demographics (age, gender, race, ethnicity, geography) from public health data and electronic health records. | U.S. Census data; CDC databases; institutional data warehouses; ensure data includes social determinants of health [19]. |
| 2. Site Selection | Choose clinical sites based on their patient population's alignment with the mapped epidemiology, including sites that serve diverse and underserved communities. | Community health centers; academic medical centers; VA hospitals; assess site cultural competency [19]. |
| 3. Outreach & Consent | Develop multilingual, culturally appropriate materials and consent forms. Use community-based participatory research principles. | Patient advocacy groups; community advisors; plain language guides; digital and non-digital outreach [18]. |
| 4. Barrier Mitigation | Implement practical support for participants: transportation vouchers, flexible scheduling, compensation for time and travel. | Budget for participant reimbursements; childcare services; virtual visit options where scientifically valid. |
| 5. Monitoring & Reporting | Continuously track enrollment demographics against pre-defined targets. Report progress to the IRB and study leadership. | Centralized tracking system; regular diversity reports; adaptive strategies if targets are not met [19]. |
The Researcher's Toolkit for Equitable Research
Table: Essential Reagents and Solutions for Implementing Justice
| Tool / Resource | Function & Application |
|---|---|
| IRB (Institutional Review Board) | An independent committee that reviews and monitors research to protect the rights and welfare of human subjects. Ensures equitable selection of subjects [17] [1]. |
| Informed Consent Form | A critical document that ensures respect for persons by providing prospective subjects with all necessary information in an understandable format, allowing them to make a voluntary decision about participation [1]. |
| Community Advisory Board | A group of community representatives that provides input on study design, recruitment strategies, and consent materials to ensure cultural and ethical appropriateness. |
| Data Diversity Dashboard | A real-time tracking tool (e.g., table, chart) that monitors enrollment demographics against pre-defined targets to ensure the study population is representative [19]. |
| Centralized IRB | For multi-site trials, a central IRB can help ensure consistent application of ethical standards and equitable subject selection practices across all sites [19]. |
Data Analysis and Presentation for Justice
Summarizing and Comparing Participant Data
A fundamental step in demonstrating justice is the clear presentation of study population characteristics. This allows reviewers to assess the generalizability of findings and the fairness of subject selection [20]. For quantitative data comparing groups, appropriate numerical summaries and graphs are essential [21].
Table: Participant Demographic and Baseline Characteristics
| Characteristic | Overall (N=85) | Group A (n=26) | Group B (n=59) | Difference (A - B) |
|---|---|---|---|---|
| Mean Age (years) | 40.2 | 45.0 | 38.1 | 6.9 |
| Median Age (years) | 37.0 | 46.5 | 35.0 | -- |
| Std. Deviation (Age) | 13.90 | 14.04 | 13.44 | -- |
| Mean Household Size | 8.4 | 10.5 | 7.5 | 3.0 |
| Median Household Size | 7.0 | 8.5 | 6.0 | -- |
| IQR (Household Size) | 6.00 | 7.75 | -- | -- |
Source: Adapted from example data on household characteristics [21]
Visualizing Distributions for Comparison
Effective data visualization is key to communicating distribution and comparisons. The choice of graph depends on the data type and the story to be told [22] [20].
- Boxplots: Best for comparing distributions across groups, showing medians, quartiles, and potential outliers. They are excellent for visualizing the center and spread of data [21] [20].
- Bar Charts: Ideal for comparing summary statistics (e.g., mean values) between discrete categories [22].
- Histograms: Used to show the frequency distribution of a continuous variable, helping to assess the shape of the data [22].
The following workflow diagram guides the selection of an appropriate comparative visualization based on the data structure and objective, ensuring clear and ethical presentation of study populations and outcomes.
The principle of justice, as articulated in the Belmont Report, remains a vital and dynamic force in shaping ethical research conduct. For today's researchers and drug development professionals, moving beyond mere regulatory compliance to a proactive commitment to justice is imperative. This involves designing studies with equitable inclusion criteria, implementing robust strategies to recruit representative samples, and transparently reporting participant demographics. By rigorously applying these principles and methodologies, the research community can ensure that the benefits and burdens of science are shared fairly, fostering trust and advancing health equity for all.
From Theory to Practice: Applying Belmont Report Principles in Clinical Research and Drug Development
The Belmont Report, formulated in 1979, established an enduring ethical framework for research involving human subjects. Its creation was a direct response to ethical transgressions in studies such as the Tuskegee Syphilis Study, where participants were denied treatment and deceived for decades [23] [24]. The report articulates three fundamental ethical principles: Respect for Persons, Beneficence, and Justice [2] [25]. Of these, the principle of Respect for Persons forms the foundational imperative for the informed consent process, requiring that individuals are treated as autonomous agents and that those with diminished autonomy are entitled to protection [2]. This guide provides a technical roadmap for researchers and drug development professionals to operationalize this principle, translating ethical theory into rigorous, respectful, and effective research practice.
The Ethical and Regulatory Foundations of Informed Consent
The Principle of Respect for Persons
The Belmont Report breaks down the principle of Respect for Persons into two key tenets: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection [2]. The application of this principle is not merely a regulatory hurdle; it is a critical factor in building and maintaining trust in clinical research [26]. When participants feel respected, they are more likely to engage, remain in studies, and contribute to the integrity of the research data.
Informed consent is the primary mechanism through which respect for persons is realized. It is fundamentally a process—not a single event or a form to be signed—that begins with the initial recruitment and continues throughout the participant's involvement in the study [27]. The Belmont Report specifically defines informed consent as being comprised of three critical elements: information, comprehension, and voluntariness [23]. This framework ensures that consent is not just legally documented but is meaningfully given.
From Belmont to Regulation: The Requirements for Consent
The ethical principles of the Belmont Report are codified in the Federal Regulations (45 CFR 46), which provide the specific elements that must be included in the informed consent process and documentation [25] [24]. The 2018 revision to the Common Rule emphasized the need for a "concise and focused" presentation of key information at the beginning of the consent document to help potential participants understand why they might or might not want to take part [27].
Table: Key Information Elements for Informed Consent as per the Revised Common Rule
| Element Number | Description of Key Information element |
|---|---|
| 1 | A statement that the project is research and that participation is voluntary. |
| 2 | A summary of the research (purpose, duration, and procedures). |
| 3 | A description of reasonably foreseeable risks or discomforts. |
| 4 | A description of reasonably expected benefits. |
| 5 | A disclosure of appropriate alternative procedures or courses of treatment, if any. |
Beyond these key elements, the regulations stipulate additional required elements for full disclosure, such as how confidentiality will be maintained, the availability of compensation for injuries, whom to contact for questions, and the conditions under which participation may be terminated [27].
A Framework for Operationalizing Respect
Moving from regulatory checkboxes to a meaningful consent process requires a deliberate strategy. The following framework, derived from ethical principles and empirical research, outlines this progression.
Element 1: Providing Sufficient Information
The first element requires that subjects are provided with all information material to their decision. This goes beyond simply listing risks and benefits; it involves presenting a complete and honest picture of the research. Researchers must avoid technical jargon and present information in straightforward language that is understandable to the participant population, typically at an 8th-grade reading level [27]. A best practice is to have a colleague or friend read the informed consent document for comprehension before it is submitted for IRB review [27].
Element 2: Ensuring Participant Comprehension
The Belmont Report states that "the manner and context in which information is conveyed is as important as the information itself" [23]. Simply providing information is insufficient if the participant does not understand it. Researchers must actively facilitate comprehension by considering the subject's maturity, capacity for understanding, language, and literacy [23]. This can involve:
- Allowing ample time for the subject to consider the information [23].
- Using comprehension tests or teach-back methods, where participants explain the study in their own words [23].
- Employing multimedia aids or culturally and linguistically appropriate materials.
This is particularly crucial when working with populations that may have diminished autonomy or cognitive impairments, where extra protections and adapted communication strategies are necessary [28] [2].
Element 3: Guaranteeing Voluntariness
An agreement to participate is only valid if it is made voluntarily, free from any form of coercion or undue influence [23] [25]. This means researchers cannot threaten harm or offer an "excessive, unwarranted, inappropriate or improper reward" to obtain compliance [23]. Special care must be taken when enrolling vulnerable individuals who are under the authority of others, such as prisoners, employees, or the very sick [28] [23]. The consent process must make it clear that participation is entirely voluntary and that the participant can withdraw at any time without penalty or loss of benefits to which they are otherwise entitled [25] [24].
Practical Protocols and Methodologies
Protocol for Developing and Assessing a Consent Document
A rigorous, multi-step protocol ensures the consent document is both ethically sound and practically effective.
Table: Protocol for Consent Document Development and Assessment
| Step | Action | Rationale & Measurement |
|---|---|---|
| 1. Drafting | Use an institutional template that includes all Common Rule elements [27]. | Ensures regulatory compliance. |
| 2. Tailoring | Adapt language, examples, and reading level to the specific subject population [27]. | Enhances relevance and accessibility. |
| 3. Internal Review | Have a colleague unfamiliar with the study read for comprehension [27]. | Identifies jargon and complex sections. |
| 4. Pilot Testing | Test the document with a small group representative of the study population. | Gathers feedback on clarity and understanding. |
| 5. Revision | Incorporate feedback to simplify and clarify the document. | Improves overall quality and effectiveness. |
| 6. IRB Submission | Submit the final document and process description for ethics review. | Fulfills institutional and federal oversight requirements. |
Methodology for a Patient-Centered Consent Process
Recent research emphasizes moving beyond a one-size-fits-all approach. A 2023 modified Delphi study engaged patients to identify what they value most in the consent process [29]. The study found that participants prioritized two key areas:
- The provision of study information to support decision-making.
- Interactions with research staff characterized by kindness, patience, and a lack of judgment [29].
This patient-centered methodology can be integrated into standard practice by:
- Training research staff in empathetic communication and active listening skills.
- Structuring the consent conversation as a two-way dialogue rather than a monologue.
- Creating a comfortable environment where questions are encouraged and answered patiently.
The Scientist's Toolkit: Essential Materials for Ethical Consent
Successfully implementing a meaningful consent process requires more than just a form. The following toolkit comprises essential resources for the modern researcher.
Table: Research Reagent Solutions for the Informed Consent Process
| Tool or Material | Function & Application |
|---|---|
| IRB-Approved Consent Template | Pre-formatted document (e.g., from university HRPP office) ensuring all required regulatory elements and institutional language are included [27]. |
| Plain Language Guidelines | Resources (e.g., plainlanguage.gov) to help translate complex scientific and technical terms into language accessible to a lay audience [27]. |
| Readability Analyzer | Software tool (e.g., in Microsoft Word) to objectively assess and target an 8th-grade reading level in the consent document [27]. |
| Multi-Media Aids | Videos, diagrams, and interactive digital platforms to supplement written text and cater to different learning styles. |
| Teach-Back Script | A structured guide for researchers to ask participants to explain the study in their own words, verifying true comprehension [23]. |
| Cultural & Linguistic Resources | Access to certified translation services and cultural consultants to ensure the process is appropriate for diverse populations. |
Addressing Contemporary Challenges and Vulnerabilities
Navigating Vulnerability with an Analytical Approach
While early ethical guidelines often employed a "group-based" or "labeling" approach to vulnerability (e.g., classifying all prisoners or children as vulnerable), modern scholarship advocates for a more nuanced "analytical approach" [28]. This approach focuses on identifying the specific sources of vulnerability in a research context, which often fall into three categories:
- Consent-based accounts: Vulnerability stems from a compromised capacity to provide free and informed consent.
- Harm-based accounts: Vulnerability arises from a higher probability of incurring harm during research.
- Justice-based accounts: Vulnerability is linked to systemic inequalities and unfair distribution of research burdens and benefits [28].
Researchers should conduct a vulnerability analysis for their study population and implement targeted safeguards. For instance, for a participant with low health literacy (a consent-based vulnerability), the use of a teach-back method is an appropriate specific safeguard.
Building Trust through Transparency and Empowerment
Trust is not a static given; it is an emergent property that develops through complex interactions within the research ecosystem [26]. It is cultivated at multiple levels: individual (researcher-participant), team, organizational, and system-wide. Key elements for building trust include transparency, respect, autonomy, and empowerment [26]. This means:
- Being transparent about data use and study results.
- Respecting participants' time, values, and perspectives.
- Protecting their autonomy throughout the study, not just at consent.
- Empowering them with knowledge and confidence.
This is especially critical when engaging with marginalized communities that harbor a historical and justified distrust of research [29] [26]. A meaningful consent process is a powerful first step in rebuilding this trust.
A meaningful informed consent process is the practical embodiment of the Belmont Report's principle of Respect for Persons. It demands that researchers move beyond a regulatory checklist and embrace their ethical duty to ensure participants are truly informed, comprehend the research, and volunteer freely. By adopting the structured frameworks, protocols, and tools outlined in this guide—and by centering the values and perspectives of participants—researchers and drug development professionals can uphold the highest ethical standards. This commitment not only protects participants but also enhances the scientific validity and societal value of the research itself.
The conduct of ethical research is anchored in the systematic assessment of risks and benefits. This process is fundamentally guided by the three core principles established by the Belmont Report: Respect for Persons, Beneficence, and Justice [1]. This guide provides researchers, scientists, and drug development professionals with a technical framework for integrating these principles into their study protocols through a rigorous and transparent risk-benefit assessment. Adherence to this framework is essential for protecting human subjects and ensuring the scientific and ethical integrity of clinical research.
Core Ethical Principles and Their Application to Risk-Benefit Assessment
The Belmont Report's principles provide the ethical justification for risk-benefit assessments. The following table outlines their core tenets and direct application to protocol development.
Table 1: Applying Belmont Report Principles to Risk-Benefit Assessment
| Ethical Principle | Core Tenet | Application in Study Protocol Design |
|---|---|---|
| Respect for Persons | Recognizing participant autonomy and protecting those with diminished autonomy [1]. | - Obtaining informed consent through a complete disclosure of risks, benefits, and alternatives.- Implementing robust data privacy and confidentiality safeguards. |
| Beneficence | The obligation to maximize possible benefits and minimize possible harms [1]. | - Designing a sound scientific protocol to ensure the research is valid and valuable.- Implementing risk minimization strategies (e.g., safety monitoring, data safety monitoring boards). |
| Justice | The fair distribution of the burdens and benefits of research [1]. | - Ensuring the selection of subjects is equitable and does not target vulnerable populations without justification.- Defining inclusion/exclusion criteria based on the scientific goals, not administrative convenience. |
A Structured Workflow for Risk-Benefit Assessment
A systematic assessment requires a structured, multi-step process. The diagram below outlines the key sequential steps for which investigators bear primary responsibility, leading to the IRB's ultimate determination.
Quantitative Frameworks for Benefit-Risk Assessment
While a qualitative summary of benefits and risks is common, a shift toward more quantitative approaches is occurring to enhance objectivity, transparency, and reproducibility [30]. A robust quantitative framework should be as quantitative as possible, incorporate the patient's perspective, be transparent, and be applicable throughout the drug's lifecycle [30].
Core Quantitative Metrics and Data Presentation
Systematic reviews and protocols should use an information-preserving approach, reporting absolute risks to allow for the calculation of various metrics [31]. The table below summarizes key quantitative metrics used in benefit-risk assessments.
Table 2: Key Quantitative Metrics for Benefit-Risk Assessment
| Metric | Description | Application & Interpretation |
|---|---|---|
| Absolute Risk (AR) | The actual event rate in a population (e.g., events per 1,000 person-years) [31]. | Serves as the foundational data point for calculating other metrics. Must be reported for both treatment and control groups. |
| Risk Difference (RD) | The absolute difference in event rates between two groups. | Provides a direct measure of the intervention's effect size. Calculated from absolute risks. |
| Number Needed to Treat (NNT) | The number of patients needing treatment for one to benefit. NNT = 1 / RD (for benefit). | A clinically intuitive measure for benefits. A lower NNT indicates a more effective intervention. |
| Number Needed to Harm (NNH) | The number of patients needing treatment for one to be harmed. NNH = 1 / RD (for harm). | A clinically intuitive measure for risks. A higher NNH indicates a safer intervention. |
| NNT to NNH Ratio | A simple ratio comparing the metrics for a key benefit and a key harm [31]. | Provides a single quantitative value. A ratio >1 suggests benefits may outweigh the specific harm, but requires context of severity. |
An Advanced Quantitative Framework
A more sophisticated quantitative framework aims to create a true benefit-risk ratio by incorporating the severity of outcomes and the context of the disease. One proposed model uses the following equation [30]:
Benefit-Risk Ratio = (Frequency of Benefit × Severity of Disease) / (Frequency of Adverse Reaction × Severity of Adverse Reaction)
This model requires operational definitions for severity. The Common Terminology Criteria for Adverse Events (CTCAE), which grades adverse events based on their impact on Activities of Daily Living (ADL), provides a validated methodology for quantifying severity [30]. This allows for a more nuanced comparison than frequency alone.
Methodologies for Key Assessment Experiments
Conducting a Systematic Literature Review for Benefits and Harms
A systematic review to inform a benefit-harm assessment should follow a pre-defined protocol [31]. Key methodological steps include:
- Identify Key Outcomes: Pre-specify the critical potential benefits and harms, prioritizing patient-important health outcomes [31].
- Comprehensive Search Strategy: Use electronic databases (e.g., MEDLINE, EMBASE) and manual searches of grey literature from health technology assessment (HTA) bodies and regulatory agencies to minimize bias [32] [31].
- Data Extraction and Synthesis: Preserve information by extracting event rates for each outcome in each treatment group. This allows for the calculation of both absolute and relative effects [31].
- Meta-analysis: Where appropriate, statistically combine results from multiple studies to provide more precise estimates of effect for both benefits and harms.
- Assess Certainty of Evidence: Grade the strength of evidence (e.g., using GRADE) for each important benefit and harm to convey uncertainty in the assessment [31].
Systematic reviews and protocol designs should state whether preferences were considered and describe how they were ascertained [31]. Methodologies include:
- Systematic Searches: Conduct systematic searches for existing studies on patient preferences for relevant outcomes.
- Preference Elicitation Techniques: Use formal techniques such as utility surveys or discrete choice experiments to quantitatively measure how patients value different benefits and trade them off against specific risks.
- Sensitivity Analysis: Incorporate the variation in patient preferences into quantitative models to determine how the benefit-risk balance might change for individuals with different values [31].
The Scientist's Toolkit: Essential Reagents for Assessment
Table 3: Key Research Reagent Solutions for Risk-Benefit Assessment
| Tool / Reagent | Function in Risk-Benefit Assessment |
|---|---|
| Common Terminology Criteria for Adverse Events (CTCAE) | Provides a standardized grading scale (Grade 1-5) for the severity of adverse events, based on impact on Activities of Daily Living (ADL), enabling quantitative comparison [30]. |
| Structured Benefit-Risk Framework (BRF) | A quantitative or qualitative method (e.g., BRAT, PRoACT-URL) for arranging data in a standardized format to assist transparent decision-making [32] [30]. |
| Systematic Review Software (e.g., RevMan, Covidence) | Platforms that facilitate the management of literature searches, data extraction, and meta-analysis, which are foundational for evidence-based assessments [32] [31]. |
| Data Safety Monitoring Plan (DSMP) | A pre-defined plan for ongoing safety monitoring, which may include a Data Safety Monitoring Board (DSMB) to review accumulating data and recommend study modifications or termination [33]. |
| Patient Preference Elicitation Tools | Surveys or interview guides designed to quantitatively capture how patients value different health outcomes, ensuring the assessment reflects the patient perspective [31] [30]. |
Data Visualization for Effective Communication
Clear presentation of benefit-risk data is crucial for transparency. Adhere to the following principles:
- Clarity over Complexity: Prioritize clarity by removing unnecessary elements and ensuring labels are clear and concise [34].
- Strategic Color Use: Use color to create associations and highlight important information. Employ a single color gradient for continuous data (e.g., severity scales) and contrasting colors for comparisons (e.g., treatment vs. control). Avoid using more than seven colors and ensure sufficient contrast for accessibility [35] [36].
- Appropriate Chart Selection:
- Bar Charts: Ideal for comparing absolute risks or incidence rates across different categories (e.g., adverse event types) [34].
- Line Charts: Effective for displaying trends over time, such as the cumulative incidence of a benefit or harm [34].
- Forest Plots: The standard for presenting meta-analyzed estimates of effect for multiple outcomes, showing both point estimates and confidence intervals.
The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research, publicly listed in the Federal Register in April 1979, established a foundational ethical framework for research involving human subjects [3]. This report delineates three core ethical principles: Respect for Persons, Beneficence, and Justice [3]. The principle of Justice demands a fair distribution of the burdens and benefits of research, compelling us to examine whether vulnerable populations are being systematically selected for research due to their availability or compromised position, rather than for reasons directly related to the problem being studied [3]. In the context of recruitment, this principle mandates that the selection of research subjects must be scrutinized to ensure that no population is unjustly burdened or excluded without a scientifically valid and ethically sound reason.
This guide provides a practical framework for researchers, scientists, and drug development professionals to implement the principle of Justice in their recruitment strategies. The goal is to move beyond theoretical adherence to operationalizing equity, ensuring that subject selection is conducted fairly and that the resulting data is robust and generalizable. By embedding these strategies into experimental protocols, the scientific community can uphold its ethical commitments while enhancing the quality and impact of its research.
The Belmont Report's Principle of Justice and Its Applications
Historical Context and Ethical Imperative
The Justice principle emerged from a historical context where ethically questionable research had disproportionately targeted vulnerable populations [3]. The Belmont Report specifically addresses the selection of subjects, highlighting the injustice of involving populations who are not in a position to refuse participation, while the benefits of the research flow primarily to more advantaged social groups [3]. This principle compels researchers to ask: "Why is this particular population being chosen for this research? Are the populations that bear the risks of research the same ones that will enjoy its benefits?"
From Principle to Practice in Subject Selection
Translating the Justice principle into recruitment practices involves proactive strategies to prevent the systematic exclusion or over-representation of specific groups. This requires a deliberate move from convenience sampling to equitable inclusion. The following table outlines common ethical challenges in recruitment and their corresponding just solutions, grounded in the Belmont framework.
Table 1: Ethical Challenges and Just Solutions in Subject Recruitment
| Ethical Challenge | Principle of Justice Applied | Just Recruitment Solution |
|---|---|---|
| Systematic Exclusion: Over-reliance on easily accessible populations (e.g., students, institutionalized individuals), leading to non-generalizable data and unfair burden on these groups. | The benefits and burdens of research should be distributed fairly across society. | Implement purposive sampling strategies that intentionally include diverse populations reflective of the disease prevalence or treatment use in the broader community [3]. |
| Exploitation of Vulnerable Groups: Recruiting from "captive" or economically disadvantaged populations due to their inability to refuse. | Individuals or groups should not be selected for research due to their ease of availability, compromised position, or manipulability. | Establish robust informed consent processes that are culturally and linguistically appropriate. Ensure that participation is truly voluntary and not coerced by perceived benefits or authority figures [3]. |
| Lack of Access to Beneficial Research: Denying potentially beneficial research to certain groups based on race, ethnicity, gender, age, or disability. | All qualified individuals should have an equal opportunity to participate in and benefit from research. | Design inclusive eligibility criteria that are as broad as scientifically possible, avoiding unnecessary restrictions that historically lead to the exclusion of certain groups (e.g., women of childbearing potential) [3]. |
Quantitative and Qualitative Frameworks for Equitable Recruitment
Ensuring justice in recruitment requires a mixed-methods approach that leverages both quantitative and qualitative research methodologies. This synergy allows researchers to measure recruitment outcomes statistically while also understanding the underlying reasons for participation or refusal among different demographic groups [37] [38] [39].
Quantitative Methodology: Measuring Equity with Data
Quantitative methodology uses numerical data to uncover patterns, trends, and disparities in recruitment. This approach is essential for objectively monitoring whether recruitment goals for diverse subgroups are being met [37] [39].
- Data Collection Methods: Structured tools such as recruitment tracking surveys and electronic data capture (EDC) systems are used to collect quantifiable data on participant demographics, enrollment rates, and reasons for screen failures [38] [39]. This data is typically gathered from a large sample population to ensure statistical power.
- Data Analysis: The strength of quantitative methodology lies in its ability to facilitate statistical analysis [37] [39]. Researchers can use statistical tests to identify significant disparities in enrollment rates across different demographic segments. This allows for data-driven decisions on where to focus recruitment efforts to achieve equity.
Table 2: Quantitative Metrics for Monitoring Equitable Recruitment
| Metric | Function | Measurement Frequency |
|---|---|---|
| Demographic Enrollment Ratios | Compares the proportion of a demographic group enrolled in the study to its proportion in the community disease population. | Bi-weekly |
| Screen Failure Analytics | Analyzes reasons for screen failures (e.g., eligibility criteria, consent withdrawal) disaggregated by key demographics. | Monthly |
| Time-to-Enrollment by Subgroup | Tracks the average time required to enroll participants from different demographic backgrounds, identifying systemic barriers. | Quarterly |
| Pre-screener Survey Data | Uses structured, closed-ended questions to quickly assess initial interest and eligibility from a broad pool [39]. | Continuously |
Qualitative Methodology: Understanding Context and Barriers
Qualitative methodology delves into the underlying motivations, perceptions, and experiences of potential and enrolled participants [38] [39]. It answers the "why" behind the quantitative data, providing rich, contextual insights that numbers alone cannot [37].
- Data Collection Methods: This approach utilizes in-depth interviews and focus groups with community members and participants to gather non-numerical, descriptive data [38] [39]. These methods are particularly valuable for exploring sensitive topics like trust in the medical system and perceived risks of participation.
- Data Analysis: Data from interviews and focus groups are analyzed through thematic analysis, which involves interpreting the data to identify common themes and patterns [38] [39]. This process helps uncover hidden insights and the nuanced reasons behind healthcare and research participation decisions [39].
The following diagram illustrates the strategic workflow for integrating quantitative and qualitative methods to achieve equitable recruitment, grounded in the Belmont principle of Justice.
Experimental Protocols and Monitoring for Equity
Detailed Protocol for Community Engagement
Objective: To build trust and understand perceived barriers to research participation within communities underrepresented in clinical research, prior to finalizing study design.
Methodology:
- Community Advisory Board (CAB) Formation: Establish a CAB of 8-12 members representing the target community's diversity (e.g., age, gender, socioeconomic status, past research experience).
- Semi-Structured Interview Guides: Develop open-ended questions to explore:
- Historical and contemporary trust levels with research institutions.
- Perceived risks and benefits of participating in clinical trials.
- Practical barriers (transportation, childcare, work schedules).
- Preferences for communication and informed consent processes.
- Data Collection: Conduct focus groups (n=4-6 groups) and individual interviews (n=15-20) until thematic saturation is reached. Sessions should be audio-recorded, transcribed, and anonymized.
- Data Analysis: Employ a thematic analysis approach [38] [39]. This involves:
- Familiarization: Reading and re-reading transcripts.
- Coding: Generating initial codes from the data.
- Theme Development: Collating codes into potential themes.
- Theme Review: Refining themes to ensure they accurately represent the data set.
- Theme Definition: Finalizing and naming themes to describe the central barriers and facilitators to participation.
Outcome Integration: The findings from this qualitative research should be used to adapt the study's recruitment materials, consent forms, and protocol logistics (e.g., offering flexible visit hours, providing travel stipends) to mitigate identified barriers.
Protocol for a Randomized Controlled Trial of Recruitment Materials
Objective: To quantitatively test the effectiveness of different recruitment material formats on enrollment rates across diverse demographic subgroups.
Methodology:
- Design: A randomized controlled trial (RCT) embedded within the broader study recruitment.
- Interventions: Develop multiple versions of recruitment materials (e.g., brochures, online ads) that vary in:
- Visuals: Imagery representing different racial/ethnic groups.
- Messaging: Framing that emphasizes community benefit vs. personal benefit.
- Readability: Simplified language (≤ 8th-grade level) vs. standard language.
- Randomization: Potential participants identified through pre-screeners are randomly assigned to view one version of the material.
- Outcome Measures:
- Primary: Rate of progression to full screening, disaggregated by demographic data (age, gender, race, ethnicity) collected in the pre-screener.
- Secondary: Time from first contact to consent, and screen failure reasons.
- Statistical Analysis: Use chi-square tests to compare enrollment rates between material types within each demographic subgroup. A p-value of <0.05 will be considered statistically significant. This statistical analysis allows for identifying which material resonates most with which group [37] [39].
The Scientist's Toolkit: Key Reagent Solutions for Equitable Research
Implementing equitable recruitment requires not only ethical commitment but also the right tools. The following table details essential "research reagents" and resources for building a just recruitment strategy.
Table 3: Research Reagent Solutions for Equitable Recruitment
| Tool / Resource | Function | Application in Ensuring Justice |
|---|---|---|
| Community Advisory Board (CAB) | A group of community stakeholders who provide guidance on research design, recruitment, and conduct. | Ensures research is culturally appropriate and addresses community concerns, preventing exploitation and building trust [3]. |
| Multilingual Consent Documentation | Informed consent forms and materials translated and back-translated into key languages spoken by the community. | Upholds Respect for Persons by ensuring non-English speakers can make a truly informed decision about participation [3]. |
| Digital Recruitment Analytics Platform | Software that tracks recruitment metrics in real-time, with disaggregation by demographics. | Allows for continuous quantitative monitoring of enrollment equity, enabling rapid corrective action if disparities arise [37] [39]. |
| Cultural Liaison or Patient Navigator | An individual who acts as a bridge between the research team and the participant community. | Helps navigate logistical and cultural barriers, reducing dropout rates and making participation more accessible for all [40]. |
| Pre-screener Surveys | Structured, closed-ended questionnaires to quickly assess initial eligibility from a large pool [39]. | Provides initial quantitative data on the diversity of the applicant pool before the main study screening, helping to identify potential biases early. |
Upholding the Belmont Report's principle of Justice in subject recruitment is an active and ongoing process that requires deliberate strategy, methodological rigor, and a commitment to self-evaluation. By integrating quantitative monitoring with qualitative understanding, and by designing protocols that are inclusive from their inception, researchers and drug development professionals can ensure that the burdens and benefits of research are shared fairly. This approach not only fulfills our ethical obligations but also produces more rigorous, generalizable, and impactful science. As the field evolves, so too must our strategies, ensuring that the foundational principles of Respect for Persons, Beneficence, and Justice remain at the core of all research involving human subjects [3].
Institutional Review Boards (IRBs) serve as a cornerstone of ethical research, providing independent review to protect the rights and welfare of human subjects [41] [42]. The Belmont Report, published in 1979, established the three fundamental ethical principles that form the analytical framework for this oversight: Respect for Persons, Beneficence, and Justice [43] [1]. For researchers, scientists, and drug development professionals, understanding how IRBs systematically apply these principles is crucial for designing ethically sound and approvable research protocols. This guide details the practical methodology IRBs use to translate the Belmont Report's ethical framework into a rigorous review process.
The Foundation: Core Ethical Principles of the Belmont Report
The Belmont Report originated from the work of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, formed in the wake of ethical abuses such as the Tuskegee Syphilis Study [43] [3]. It was created to identify comprehensive ethical principles and guidelines for the protection of human subjects [3]. The report establishes three principles that now serve as the primary ethical basis for the U.S. system of human research protection [1].
The following table summarizes the three core principles and their core ethical convictions:
Table 1: Foundational Ethical Principles of the Belmont Report
| Principle | Ethical Convictions | Moral Requirements |
|---|---|---|
| Respect for Persons | Individuals should be treated as autonomous agents; persons with diminished autonomy are entitled to protection [1]. | Acknowledgement of autonomy through voluntary consent; protection of those with diminished autonomy [1]. |
| Beneficence | Persons are treated ethically by securing their well-being [1]. | Do not harm; maximize possible benefits and minimize possible harms [1]. |
| Justice | The benefits and burdens of research must be distributed fairly [43] [1]. | Fair selection of subjects to avoid exploiting vulnerable or easily available populations [1]. |
The Analytical Framework: Applying Belmont Principles in IRB Review
The Belmont Report provides the ethical foundation, while IRBs serve as the practical application mechanism. An IRB is an appropriately constituted group formally designated to review and monitor biomedical research involving human subjects [41]. Its purpose is to assure that appropriate steps are taken to protect the rights and welfare of humans participating in research [41].
The diagram below illustrates the logical workflow of how an IRB uses the Belmont Principles as an analytical framework for protocol review.
Application of Respect for Persons: The Informed Consent Process
The principle of Respect for Persons requires that individuals enter research voluntarily and with adequate information [1]. During review, the IRB scrutinizes the informed consent process and documentation to ensure this principle is upheld.
IRB Review Methodology for Informed Consent:
- Comprehensiveness Review: The IRB verifies that the consent document includes all essential elements as required by regulations (e.g., research procedures, purposes, risks, benefits, alternatives) [1].
- Comprehensibility Assessment: The board ensures the information is presented in a language and at a level that is understandable to the prospective subject [1].
- Voluntariness Safeguards: The IRB reviews the process for obtaining consent to ensure it is free from coercion or undue influence [1].
- Vulnerable Populations Protection: For subjects with diminished autonomy, the IRB assesses the adequacy of additional safeguards, such as the use of legally authorized representatives [1].
Application of Beneficence: Systematic Risk-Benefit Assessment
The principle of Beneficence requires that researchers not only protect subjects from harm but also maximize potential benefits and minimize potential risks [1]. The IRB's review involves a systematic and non-arbitrary method to determine if the research risks are justified.
IRB Review Methodology for Risk-Benefit Assessment:
- Risk Identification and Minimization: The IRB requires that "risks to subjects are minimized" using sound research design and procedures [42].
- Risk Reasonableness Determination: The board evaluates whether the "risks are reasonable in relation to the importance of the knowledge the study is expected to produce" [42]. This involves a careful analysis of the scientific validity and potential value of the research.
- Benefit Analysis: The IRB assesses the anticipated benefits to the subject or to society, ensuring they are maximized where possible [1].
Application of Justice: Ensuring Equitable Subject Selection
The principle of Justice requires that the selection of research subjects be distributed fairly, ensuring that the burdens of research do not fall disproportionately on vulnerable groups while the benefits flow to more privileged populations [1].
IRB Review Methodology for Subject Selection:
- Inclusion/Exclusion Criteria Scrutiny: The IRB reviews the criteria for including or excluding subjects to ensure they are based on the scientific goals of the research and not merely on the easy availability, compromised position, or manipulability of certain groups [1].
- Vulnerability Assessment: The board examines whether there are "adequate provisions to protect vulnerable populations from coercion or undue influence" [42]. The IRB membership must include knowledge about any vulnerable groups that are regularly reviewed [42].
- Distributional Equity Evaluation: The IRB ensures that the research does not systematically select subjects because of racial, sexual, economic, or cultural biases [1].
The IRB's Operational Toolkit: Membership and Regulatory Authority
For an IRB to effectively apply the Belmont framework, it must be properly constituted and operate under a clear regulatory mandate.
Table 2: IRB Composition and Operational Requirements (21 CFR 56 & 45 CFR 46)
| Requirement | Regulatory Specification | Purpose in Upholding Belmont Principles |
|---|---|---|
| Membership Diversity | At least 5 members of varying backgrounds, both sexes, and more than one profession [42]. | Ensures diverse perspectives for evaluating respect, beneficence, and justice. |
| Expertise | Includes at least one scientific member, one non-scientific member, and one member unaffiliated with the institution [41] [42]. | Scientific review assesses beneficence; non-scientific and unaffiliated members bolster respect for persons and justice. |
| Vulnerable Populations | Includes knowledge about any regularly researched vulnerable groups [42]. | Directly supports the Justice principle in subject selection. |
| Quorum & Voting | A quorum of a majority at convened meetings; approval requires a majority vote [42]. | Ensures collective, group-based deliberation on ethical issues. |
| Conflict of Interest | Members must recuse themselves from review of projects in which they have a conflicting interest [41]. | Protects the integrity and independence of the review process. |
The FDA regulations empower IRBs to "approve, require modifications in (to secure approval), or disapprove research" [41]. This authority is central to ensuring that the principles of the Belmont Report are not merely advisory but are actively enforced in the research landscape.
The Researcher's Toolkit: Essential Documentation for IRB Submission
To facilitate a smooth IRB review, researchers must prepare a submission that explicitly addresses each Belmont principle. The following items constitute the essential "research reagents" for navigating the ethical review process.
Table 3: Essential Components of an IRB Submission
| Submission Component | Function & Purpose | Key Considerations |
|---|---|---|
| Research Protocol | The scientific blueprint of the study. Provides the necessary detail for the IRB to assess the risks and benefits (Beneficence) and the fairness of subject selection (Justice). | Must clearly justify the scientific design, methodology, and statistical rationale. |
| Informed Consent Document(s) | The primary tool for ensuring Respect for Persons. Provides prospective subjects with the information required for an autonomous decision. | Must be written in lay language, typically at an 8th-grade reading level; must include all required regulatory elements. |
| Investigator's Brochure | Summarizes the clinical and non-clinical data on the investigational product. Allows the IRB to assess potential risks and benefits (Beneficence). | For drug/device trials, this is critical for understanding the product's safety profile. |
| Recruitment Materials | All advertisements, scripts, and flyers used to enroll subjects. The IRB reviews these to ensure they are not coercive and fairly target the population (Justice and Respect for Persons). | Materials should not promise undue benefits or minimize risks. |
| Investigator CV & Credentials | Demonstrates the investigator and site are qualified to conduct the research safely and ethically (Beneficence). | Highlights prior experience, training, and appropriate resources. |
The Belmont Report is not a historical artifact but a living framework that actively guides the protection of human research subjects. For clinical investigators and drug development professionals, a deep understanding of how IRBs analytically apply the principles of Respect for Persons, Beneficence, and Justice is indispensable. By designing research protocols that are fundamentally structured around these ethical pillars, researchers engage as partners with IRBs in the shared mission of advancing science while vigilantly safeguarding the rights and welfare of the human subjects who make this progress possible.
The Belmont Report, published in 1979, established three core ethical principles—Respect for Persons, Beneficence, and Justice—that form the foundational framework for conducting ethical scientific research involving human subjects [44]. Within pharmaceutical clinical trials, where the balance between scientific advancement and participant welfare is paramount, these principles provide critical guidance for research design and conduct. This case study examines the application of these principles within a hypothetical Phase III randomized controlled trial (RCT) for a novel Alzheimer's drug, "CogniCare," demonstrating how ethical guidelines translate into practical trial protocols. Adherence to these principles protects participants and enhances scientific validity, with modern clinical trial protocols guided by standards like the SPIRIT 2025 statement to ensure comprehensive and transparent reporting [45].
Application of Belmont Report Principles in Clinical Trial Design
The three principles of the Belmont Report directly inform specific components of clinical trial protocol development and operational execution.
Principle 1: Respect for Persons
The principle of Respect for Persons emphasizes individual autonomy and requires that participants enter into research voluntarily and with adequate information. This is operationalized primarily through the informed consent process.
- Application in Protocol Design: The trial protocol incorporates a multi-stage informed consent process. This involves clear explanations of the study's purpose, procedures, risks, benefits, and alternatives, provided in language accessible to a layperson. The protocol mandates that consent forms avoid technical jargon and that researchers assess participant understanding through teach-back methods.
- Safeguards for Vulnerable Populations: For a trial involving Alzheimer's patients, who may have diminished decision-making capacity, the protocol requires a dual-consent process. This involves obtaining consent from both the patient (to the extent of their ability) and a legally authorized representative. Assent from the patient is sought even when a representative provides formal consent.
- Ongoing Process: Consent is treated not as a single event but as a continuous process. Participants are re-consented if significant new risk information emerges during the trial, reaffirming their autonomous choice to continue.
Principle 2: Beneficence
The principle of Beneficence mandates an obligation to maximize possible benefits and minimize potential harms to research participants. This requires a systematic and rigorous assessment of risks and benefits.
- Application in Protocol Design: The trial design incorporates a robust risk-benefit analysis overseen by an independent Data and Safety Monitoring Board (DSMB). The statistical analysis plan pre-defines stopping boundaries for efficacy and futility, allowing the trial to be halted early if the drug demonstrates overwhelming benefit or if predefined safety thresholds are crossed.
- Risk Minimization Strategies: The protocol includes stringent safety monitoring procedures, such as frequent laboratory tests and imaging schedules, to promptly identify adverse events. A pharmacovigilance plan details the reporting pathways for these events to both the Institutional Review Board (IRB) and regulatory authorities.
- Benefit Maximization: The study design compares the new intervention against an active comparator (standard of care) rather than a placebo, where ethically permissible, ensuring that the control group receives a known effective treatment.
Principle 3: Justice
The principle of Justice requires the fair distribution of the benefits and burdens of research. This principle addresses the selection of subjects and ensures that vulnerable populations are not disproportionately targeted for risky research while being excluded from the benefits of its outcomes.
- Application in Protocol Design: The participant recruitment strategy is designed to ensure a diverse and representative study population. The protocol sets enrollment targets to include participants from various age groups, genders, and racial and ethnic backgrounds reflective of the broader Alzheimer's disease population.
- Avoiding Exploitation: Recruitment avoids being concentrated solely in underserved communities with limited healthcare access. The protocol includes measures to reduce participation barriers, such as providing transportation vouchers and compensating participants for their time and travel.
- Post-Trial Access: The protocol outlines a plan for post-trial access to the investigational drug for participants who benefit from it, addressing the equitable distribution of the research's positive outcomes.
Table 1: Mapping Belmont Report Principles to Clinical Trial Protocol Elements
| Belmont Principle | Protocol Section | Specific Application in "CogniCare" Trial |
|---|---|---|
| Respect for Persons | Informed Consent | Multi-stage consent with capacity assessment and a dual-consent model for patients with fluctuating cognition. |
| Beneficence | Data Safety Monitoring | Independent DSMB with pre-defined stopping rules based on efficacy and adverse event rates. |
| Justice | Eligibility & Recruitment | Enrollment targets for underrepresented groups; strategies to reduce participation barriers. |
Methodological Framework and Data Presentation
Adherence to SPIRIT 2025 Protocol Guidelines
The "CogniCare" trial protocol is developed in accordance with the SPIRIT 2025 statement, which defines standard protocol items for clinical trials to ensure transparency and completeness [45]. Key methodological components include the PICO framework (Population, Intervention, Comparator, Outcome) and a detailed statistical analysis plan. The transition from protocol-defined outcomes to reported results is critical, as registries like ClinicalTrials.gov serve as valuable repositories that can mitigate publication bias and selective reporting [46].
Structured Data Presentation of Efficacy and Safety
The presentation of clinical trial data in clear tables and graphs is crucial for accurate communication and interpretation among healthcare providers and researchers [47]. Data should be organized to allow for a dual-faceted understanding of both efficacy and safety [46].
Table 2: Efficacy Outcomes at 12-Month Follow-Up (Hypothetical Data)
| Study Arm | n | Mean Change in ADAS-Cog11 Score (Primary Outcome) | 95% Confidence Interval | p-value |
|---|---|---|---|---|
| CogniCare | 300 | -2.5 | [-3.1, -1.9] | < 0.001 |
| Active Comparator | 300 | -1.8 | [-2.4, -1.2] | — |
| Treatment Difference | — | -0.7 | [-1.5, 0.1] | 0.067 |
Table 3: Incidence of Serious Adverse Events (SAEs) > 2% (Hypothetical Data)
| Adverse Event | CogniCare (n=300) | Active Comparator (n=300) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Headache | 12 | 4.0% | 9 | 3.0% |
| Nausea | 10 | 3.3% | 6 | 2.0% |
| Dizziness | 9 | 3.0% | 5 | 1.7% |
Visual data displays, such as bar charts for participant flow or Kaplan-Meier curves for survival analysis, are employed to present data efficiently. However, research indicates that healthcare providers may not always accurately interpret complex data displays, underscoring the need for clear and well-designed visuals [48]. Formats like icon arrays, though potentially less preferred by some clinicians, have been shown to improve comprehension of risk information [48].
Visualizing the Ethical Framework and Trial Workflow
The following diagram illustrates the logical workflow of how the Belmont Report principles are integrated into the key stages of the clinical trial process.
The following diagram details the sequential workflow for implementing the beneficence principle through safety monitoring and the DSMB's independent oversight.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key reagents and materials essential for conducting a rigorous pharmaceutical clinical trial, along with their specific functions in upholding research integrity and the ethical principles outlined above.
Table 4: Essential Research Reagent Solutions for Clinical Trial Execution
| Item | Function & Application in Trial |
|---|---|
| Electronic Data Capture (EDC) System | A secure, validated software platform for collecting, managing, and cleaning clinical trial data in real time. Ensures data integrity (Beneficence) and participant confidentiality (Respect for Persons). |
| Interactive Web Response System (IWRS) | A system used to randomize participants to trial arms and manage drug inventory. Centralized randomization promotes unbiased allocation (Justice) and ensures the scientific validity of the trial. |
| Validated Biomarker Assays | Laboratory tests (e.g., ELISA, Mass Spectrometry) to measure pharmacokinetic/pharmacodynamic endpoints or confirm patient eligibility. Provides objective data for efficacy and safety analysis (Beneficence). |
| Certified Reference Standards | Physicochemical benchmarks of the active pharmaceutical ingredient (API) and key impurities. Essential for ensuring the quality, consistency, and safety of the investigational product (Beneficence). |
| Anonymized Biological Sample Kits | Pre-labeled kits for collecting and processing participant blood, tissue, or other samples. Standardizes procedures and protects participant identity by de-identifying samples (Respect for Persons). |
| Independent Data Repository | A secure, third-party database for storing the final trial dataset. Facilitates independent verification of results and sharing for secondary research, promoting transparency and scientific progress (Justice). |
The Belmont Report's principles are not abstract concepts but practical tools that actively shape the design, conduct, and dissemination of pharmaceutical clinical trials. By systematically applying Respect for Persons, Beneficence, and Justice—through rigorous informed consent, proactive safety monitoring, and equitable recruitment—the "CogniCare" case study demonstrates how ethical integrity and scientific excellence are inextricably linked. This integrated approach ensures that clinical research not only generates reliable evidence for new therapies but also steadfastly protects the rights, safety, and welfare of the human participants who make such advancements possible.
Navigating Ethical Dilemmas: Balancing and Optimizing Belmont Principles in Complex Scenarios
The Belmont Report, published in 1979, established three core ethical principles—Respect for Persons, Beneficence, and Justice—to guide the conduct of research with human subjects [3] [14] [7]. These principles were formulated in response to historical ethical scandals, such as the Tuskegee Syphilis Study, with the goal of ensuring the protection of participants' dignity, rights, and welfare [7] [44]. While this framework provides a robust foundation for ethical research, its application in practice frequently gives rise to complex situations where these principles are in tension.
One of the most persistent challenges in both research and clinical practice is the collision between the principle of Beneficence—the obligation to maximize benefits and minimize harms—and the principle of Autonomy (expressed in the Belmont Report as Respect for Persons)—the obligation to respect individuals' right to self-determination and their ability to make informed decisions about their own lives [14] [49]. This conflict is not merely theoretical; it manifests in everyday scenarios where a researcher's perception of a participant's best interest clashes with the participant's own expressed wishes. This guide provides an in-depth analysis of the nature of this conflict, framed within the Belmont Report's foundational principles, and offers structured methodologies and practical tools for researchers and drug development professionals to navigate these ethical dilemmas with rigor and integrity.
Defining the Core Principles
The Principle of Beneficence
The Belmont Report defines Beneficence as an obligation to protect subjects from harm by maximizing possible benefits and minimizing possible risks [14] [44]. This principle goes beyond simply avoiding harm; it constitutes an active commitment to promoting the well-being of research participants [49]. In practice, this requires a systematic assessment of risks and benefits to ensure that the potential for positive outcomes justifies any foreseeable harms [14] [3]. For researchers, this often translates into a sense of professional responsibility to act in the participant's best medical interest, a perspective deeply rooted in medical tradition and the Hippocratic Oath.
The Principle of Respect for Persons (Autonomy)
The principle of Respect for Persons expresses the ethical conviction that the autonomy of individuals should be respected and that persons with diminished autonomy are entitled to equal protection [14]. This principle acknowledges that individuals are autonomous agents capable of making their own informed decisions and life plans. The primary practical application of this principle is the requirement for informed consent [14] [49]. A meaningful consent process requires that prospective subjects are provided with all relevant information about a study, comprehend that information, and make a voluntary decision to participate without coercion or undue influence [14]. This ensures that participants are active agents in the research process, not merely passive subjects.
The Nature of the Conflict: Beneficence vs. Autonomy
The conflict between beneficence and autonomy arises when a researcher's or clinician's judgment of what is in a participant's best interest contradicts the participant's own autonomous choices [49]. This is not an abstract problem but a common source of ethical conflict in healthcare and research settings.
In clinical practice, this collision is frequently observed in end-of-life decisions, where a care team's desire to prolong life (beneficence) may conflict with a patient's wish to forgo life-sustaining treatments [50] [51]. Similarly, in palliative care, nurses often face dilemmas between aggressively managing symptoms and respecting a patient's refusal of certain medications or interventions [51].
Within research, this tension is inherent in the informed consent process for complex clinical trials. A researcher, believing in the potential benefit of a new investigational drug (beneficence), may feel conflicted when a eligible patient, despite understanding the potential benefits, autonomously chooses not to participate [14]. The Belmont Report itself acknowledges that its principles can conflict, noting that considerations of beneficence must often be balanced against the obligation to allow for subject autonomy [14]. A study of critical care nurses found that one of the most frequent ethical conflicts was "Having to administer treatments and/or carry out procedures without the critical patient, who is conscious, knowing their purpose and the risks involved," a situation that directly pits a clinical imperative (beneficence) against the patient's right to informed consent (autonomy) [52].
Table 1: Common Scenarios of Ethical Conflict Between Beneficence and Autonomy
| Scenario | Beneficence Concern (Acting for the Patient's Good) | Autonomy Concern (Respecting Self-Determination) | Common Context |
|---|---|---|---|
| End-of-Life Care | Initiating or continuing life-sustaining treatment to preserve life. | Honoring a patient's "Do Not Resuscitate" (DNR) order or advance directive. | ICU, Palliative Care [51] [52] |
| Treatment Refusal | Believing a specific intervention (e.g., surgery, medication) is medically necessary. | Respecting a patient's informed refusal of the recommended intervention. | Clinical Practice & Therapeutic Research [49] |
| Clinical Trial Enrollment | Believing a patient could benefit from an investigational therapy in a trial. | Accepting a patient's autonomous decision to decline participation after understanding the risks. | Drug Development & Clinical Research [14] |
| Procedural Consent | Performing a procedure believed to be in the patient's clinical interest. | Administering treatment without the conscious patient fully understanding its purpose and risks. | Critical Care Units [52] |
A Systematic Framework for Ethical Resolution
Resolving conflicts between beneficence and autonomy requires a structured, systematic approach to ethical problem-solving. The following methodology provides a step-by-step protocol for analyzing these dilemmas.
Experimental Protocol for Ethical Decision-Making
Objective: To provide a standardized, replicable method for identifying, analyzing, and resolving ethical conflicts between the principles of beneficence and autonomy in a research or clinical setting.
Methodology:
Situation Analysis & Problem Identification:
- Data Collection: Gather all relevant clinical, research, and contextual facts. This includes the participant's medical and psychological status, the nature of the research intervention, potential risks and benefits, and the participant's expressed wishes and understanding.
- Stakeholder Identification: Map all parties involved (e.g., research participant, family members, research team, clinical care providers, institution) and their respective interests, values, and concerns.
- Problem Definition: Clearly articulate the central ethical conflict. State the competing claims of beneficence and autonomy in specific terms (e.g., "The conflict is between the investigator's belief that Drug X offers direct therapeutic benefit and the participant's refusal due to fear of side effects").
Principle Clarification & Ethical Analysis:
- Identify Principles: Isolate the specific ethical principles in conflict, primarily beneficence and autonomy in this context. Consider if other principles, such as justice or non-maleficence, are also relevant [49] [53].
- Evaluate Claims: Scrutinize the strength of each principle's claim.
- For Beneficence: How certain is the potential benefit? How significant is it? What is the evidence base? Are there alternative ways to achieve the same good?
- For Autonomy: Is the participant's decision truly autonomous and informed? Is there evidence of coercion, undue influence, or impaired decision-making capacity? Has the consent process been thorough and ongoing?
Option Generation & Conflict Resolution:
- Brainstorm Courses of Action: Generate a comprehensive list of all possible actions, including compromises. Examples include: a) honoring autonomy entirely, b) attempting further persuasion with new information, c) seeking a second opinion, d) involving an ethics consultation, or e) in specific, limited circumstances, overriding autonomy based on a compelling beneficence-based justification.
- Evaluate Options: Weigh each option against the ethical principles, the participant's values, institutional policy, and regulatory standards. The goal is to find a solution that respects as much of both principles as possible.
- Select a Resolution: Choose the course of action that best resolves the conflict while maintaining the highest possible ethical standards. Document the rationale for the chosen path.
Implementation & Review:
- Act: Implement the chosen course of action with transparency and compassion.
- Monitor and Reflect: Continuously assess the outcomes of the decision. For recurring conflicts, use the experience to refine institutional policies, consent processes, and training protocols.
This decision-making pathway is visualized in the workflow below, which outlines the process from initial conflict identification through to resolution and review.
Quantitative Data on Ethical Conflicts
Empirical research helps to quantify the prevalence and nature of these ethical conflicts, particularly in high-acuity settings. Studies conducted among critical care nurses, for instance, provide valuable data on the frequency and intensity of these dilemmas.
Table 2: Frequency and Intensity of Ethical Conflicts in Critical Care Settings (Sample Data)
| Ethical Conflict Situation | Reported Frequency (Occurring at least once a month/week) | Reported Intensity / Level of Exposure | Primary Form of Conflict |
|---|---|---|---|
| Administering treatments without a conscious patient's full understanding [52] | 62.5% | Moderate | Moral Distress / Dilemma |
| Caring for a patient believed to be better suited for a general ward [52] | 62% | Moderate | Moral Distress |
| Using resources despite believing in its futility [52] | 68.6% | Information Not Specified | Moral Dilemma |
| Violation of patient privacy [52] | 76.9% | Information Not Specified | Moral Outrage |
Effectively navigating ethical conflicts requires both conceptual frameworks and practical tools. The following table details essential resources for researchers and ethics committees.
Table 3: Research Reagent Solutions: Essential Tools for Ethical Research Practice
| Tool / Resource | Function | Application in Conflict Resolution |
|---|---|---|
| Institutional Review Board (IRB) / Ethics Committee | Independent body that reviews, approves, and monitors research involving human subjects to ensure ethical standards. | Provides mandatory pre-review of protocols to identify potential beneficence/autonomy conflicts; available for consultation on complex cases [14] [54]. |
| Informed Consent Templates & Checklists | Standardized documents and tools to ensure all required elements of informed consent are consistently addressed. | Helps prevent conflicts by ensuring the autonomy of participants is respected from the outset through a comprehensive consent process [14] [44]. |
| Ethics Consultation Service | A dedicated, multidisciplinary team (e.g., ethicists, clinicians, legal experts) available to analyze acute ethical dilemmas. | Provides real-time, case-specific analysis and recommendations for resolving conflicts that arise during the course of research or clinical care. |
| Risk-Benefit Assessment Framework | A structured methodology for systematically identifying, quantifying, and comparing potential harms and benefits of a research intervention. | Provides an objective basis for the beneficence assessment, helping to determine if the research justifies the risks posed to autonomous participants [14] [53]. |
| Moral Distress Assessment Tools (e.g., ECNQ-CCV) | Validated questionnaires, such as the Ethical Conflict in Nursing Questionnaire–Critical Care Version, to measure staff exposure to ethical conflicts. | Allows institutions to monitor the ethical climate, identify recurring problems, and implement targeted support and training to mitigate staff distress [52]. |
The collision between beneficence and autonomy is an inherent and enduring challenge in human subjects research. The Belmont Report does not provide a simple hierarchy of principles but rather a framework that requires careful deliberation and balancing [14]. As research grows more complex, with advances in areas like gene therapy and research involving vulnerable populations, the application of these principles demands continued vigilance and refinement [3]. Successfully navigating these conflicts is not about "winning" an argument for one principle over another, but about engaging in a rigorous, systematic, and compassionate process that honors the spirit of both—the researcher's commitment to do good and the fundamental right of every person to choose their own path. By employing structured frameworks, leveraging available resources, and fostering an institutional culture of ethical awareness, researchers and drug development professionals can uphold the highest standards of integrity, ensuring that scientific progress never comes at the cost of human dignity.
Children represent a fundamentally vulnerable population in clinical research, necessitating specialized ethical frameworks to safeguard their welfare and rights. This vulnerability stems from their developmental immaturity, limited capacity for autonomous decision-making, and socio-legal status as dependents. Research involving children is indispensable for establishing safe and effective medical interventions for the pediatric population, yet it requires researchers to navigate complex ethical and legal frameworks to ensure the welfare of child participants [55]. The historical context of research ethics, including the Nuremberg Code developed in response to the unethical experiments conducted during World War II, underscores the critical importance of voluntary informed consent and the right to withdraw from research—principles that take on added complexity when applied to children [55]. Subsequent ethical guidance, including the Belmont Report (1979) and the Declaration of Helsinki (first adopted in 1964), further codified protections for vulnerable groups, recognizing that those with diminished autonomy are entitled to special protections [55] [1]. This guide examines the ethical challenges inherent in pediatric research through the lens of the Belmont Report's foundational principles, providing researchers with a structured framework for ethical decision-making.
Foundational Ethical Frameworks
The Belmont Report's Guiding Principles
The Belmont Report establishes three fundamental ethical principles for all human subjects research: respect for persons, beneficence, and justice. These principles provide the moral foundation for federal regulations governing human subjects research in the United States, including the Common Rule (45 CFR 46) [1].
Respect for Persons: This principle incorporates two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection. The application of this principle requires that subjects, to the degree they are capable, be given the opportunity to choose what shall or shall not happen to them. This opportunity is provided through the process of informed consent, which must be adapted for pediatric populations through parental permission and, when appropriate, child assent [1]. The principle acknowledges that autonomy is fluid in children, evolving with maturity, and therefore requires a developmentally-sensitive approach [56].
Beneficence: This principle entails an obligation to maximize possible benefits and minimize possible harms to research participants. It requires that researchers not merely refrain from inflicting harm, but that they act positively to secure the well-being of participants. For children, this necessitates a careful risk-benefit analysis where the potential benefits of research (either to the individual child or to society) must justify any risks presented by the research procedures [1] [55]. The principle of beneficence demands special vigilance in pediatric research, as children are often less able to understand and bear research risks.
Justice: This principle addresses the fair distribution of the benefits and burdens of research. It requires that researchers not systematically select subjects from groups unlikely to benefit from the research (e.g., institutionalized individuals or racial minorities) simply because of their easy availability or compromised position. In pediatric research, justice demands equitable consideration of which children bear research burdens and which stand to benefit from research findings, guarding against the exploitation of any particular pediatric subgroup [1] [55].
Regulatory Framework for Pediatric Research
In the United States, research involving children is governed by Subpart D of 45 CFR 46, which outlines specific conditions under which pediatric research may be approved by an Institutional Review Board (IRB). These regulations operationalize the ethical principles of the Belmont Report for the pediatric population [55].
Table 1: IRB Approval Categories for Pediatric Research under 45 CFR 46 Subpart D
| Regulatory Category | Risk Level | Permission & Assent Requirements | Direct Benefit to Participants? |
|---|---|---|---|
| §46.404 | Research not involving greater than minimal risk | Parental permission and child assent (when appropriate) | Not required |
| §46.405 | Research involving greater than minimal risk but presenting the prospect of direct benefit to individual subjects | Parental permission and child assent (when appropriate) | Yes - must justify that risk is justified by anticipated benefit to subjects |
| §46.406 | Research involving greater than minimal risk and no prospect of direct benefit to individual subjects, but likely to yield generalizable knowledge about the subject's disorder or condition | Parental permission and child assent (when appropriate) | No - risk must represent minor increase over minimal risk |
| §46.407 | Research not otherwise approvable which presents an opportunity to understand, prevent, or alleviate a serious problem affecting the health or welfare of children | Requires review by national expert panel and approval by the Secretary of HHS | Varies |
Core Ethical Challenges and Methodological Considerations
Navigating Autonomy and Consent
The principle of respect for persons presents unique challenges in pediatric research due to children's developing capacity for autonomy. The traditional triadic relationship involving the researcher, child, and parent/guardian creates a complex dynamic for consent processes [56].
Parental Permission: In most jurisdictions, the legal authority to provide consent for a child's participation in research rests with parents or legal guardians. This authority is based on the presumption that parents act in the best interests of their children. However, challenges arise when parental decisions may not align with the child's welfare or emerging preferences, or when parents themselves may lack adequate decision-making capacity [57]. Researchers must verify that parents possess sufficient understanding of the research procedures, risks, and potential benefits to provide meaningful permission.
Child Assent: Ethical practice requires seeking the affirmative agreement of children capable of providing it, in addition to parental permission. The process of assent should be developmentally appropriate, providing information about the research in understandable terms, describing what the child will experience, and clarifying that participation is voluntary. There is no universally agreed-upon age for assent, as capacity depends on individual developmental factors rather than chronological age alone [56]. Studies indicate that nearly half of minors believe 16 is an appropriate age for health decision-making, while parents often favor 18, suggesting the importance of individualized assessment [56].
Cultural Considerations: The application of autonomy principles must be culturally sensitive. In Western societies, informed consent is typically an individual decision, whereas in collectivistic cultures, decision-making may be group-oriented, requiring consultation with family or community leaders [11]. For example, research involving Native American communities may require tribal leader approval before seeking individual consent, respecting kinship-based decision-making structures [11].
Risk-Benefit Analysis in Pediatric Populations
Applying the principle of beneficence requires a rigorous risk-benefit assessment specific to pediatric populations. This assessment must consider both the direct therapeutic potential for individual participants and the societal value of generalizable knowledge gained [55].
Defining Minimal Risk: The concept of "minimal risk" is central to pediatric research ethics, serving as a threshold for many regulatory categories. According to 45 CFR 46, minimal risk means that "the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests." Interpretation of this standard requires careful consideration of the daily experiences of normal, healthy children or children with specific medical conditions.
Managing More Than Minimal Risk: Research presenting greater than minimal risk requires stronger justification. When the research offers direct therapeutic benefit to participants, the risk must be justified by the anticipated benefit. When no direct benefit is anticipated, the risk must represent only a minor increase over minimal risk and be likely to yield vital knowledge about the child's condition [55]. This balancing requires careful scientific and ethical judgment, often necessitating specialized IRB review.
Vulnerability within Vulnerability: Researchers must recognize that some pediatric populations experience compounded vulnerability. Justice-involved youth, for example, are vulnerable based on both age and system involvement, often coming from profiles of poverty, racial minority status, and complex risk factors [58]. Similarly, children with psychiatric illnesses may face additional vulnerabilities when parents lack capacity for medical decision-making, potentially leading to medical neglect [57]. These layered vulnerabilities demand augmented protections and careful ethical scrutiny.
Practical Applications and Researcher Toolkit
Ethical Decision-Making Framework for Researchers
The following workflow provides a structured approach for navigating ethical decisions in pediatric research, integrating Belmont Principles with regulatory requirements:
Table 2: Essential Resources for Ethical Pediatric Research
| Resource Category | Specific Tool/Guidance | Function & Application |
|---|---|---|
| Ethical Framework | The Belmont Report [1] | Provides foundational ethical principles (Respect for Persons, Beneficence, Justice) for research design and implementation. |
| Regulatory Guidance | 45 CFR 46, Subpart D [55] | Specifies federal regulatory requirements for pediatric research, including risk categories and approval conditions. |
| Consent/Assent Tools | Developmentally-Appropriate Assent Scripts | Explain research procedures in child-friendly language; should be tailored to the child's cognitive and emotional maturity. |
| Risk Assessment Tool | Systematic Risk-Benefit Analysis Framework | Structured approach to evaluate potential harms and benefits, comparing them to children's everyday experiences. |
| Cultural Competence | Cultural Humility Training [11] | Enables researchers to recognize diverse cultural norms around autonomy, consent, and family decision-making. |
| Special Population Guidance | IRB Guidelines for Justice-Involved Youth [58] | Addresses compounded vulnerabilities of specific pediatric subgroups requiring additional protections. |
Implementing Ethical Protocols: Lessons from the Field
Conducting ethical research with vulnerable populations requires both rigorous preparation and adaptive implementation. Key lessons from research practice include:
Lesson 1: Anticipate Complexity: There is no simple way to conduct studies with vulnerable populations. Research with justice-involved youth, for example, requires navigating both IRB requirements and government entity approvals, as these youth often fall under the principle of parens patriae (state as protector) [58]. A clear research plan must explicitly articulate steps to protect youths' information, identity, and confidentiality throughout the study.
Lesson 2: Protect Information Like People: When working with vulnerable populations, researchers have an ethical obligation to protect participant data with the same rigor as protecting the individuals themselves. This is particularly critical when using secondary data containing unique identifiers. Robust data security procedures—including secure data transfer, encryption, and de-identification protocols—are essential to maintain confidentiality and satisfy data proprietors [58].
Lesson 3: Adopt a Culturally Sensitive Lens: Recognizing that vulnerability is often contextual and nuanced, researchers must approach the principle of "Respect for Persons" with cultural humility. This means understanding that autonomy may be expressed differently across cultures—sometimes as individual choice, other times as collective or family decision-making [11]. Researchers should investigate cultural norms and adapt consent processes accordingly, which may involve community leader consultation or alternative consent documentation methods.
Research involving children remains ethically complex yet scientifically indispensable. The foundational principles of the Belmont Report—respect for persons, beneficence, and justice—provide an enduring framework for navigating these challenges. By implementing developmentally-appropriate assent processes, conducting rigorous risk-benefit analyses, ensuring equitable subject selection, and maintaining cultural humility, researchers can generate vital scientific knowledge while faithfully upholding their ethical obligations to protect this vulnerable population. The continuing evolution of pediatric research ethics demands ongoing dialogue between researchers, ethicists, policymakers, and communities to ensure that the pursuit of knowledge never compromises the welfare and rights of children.
Optimizing the Consent Process for Enhanced Comprehension and Voluntariness
Within the framework of clinical research, the informed consent process serves as a critical practical application of foundational ethical principles. It is the primary mechanism through which the research community demonstrates respect for individual autonomy and upholds the ethical commitments outlined in the Belmont Report [14]. An optimized consent process ensures that individuals are not merely subjects of research but are respected as voluntary partners who understand the nature of the research and the implications of their participation. This guide provides a technical and methodological roadmap for researchers and drug development professionals seeking to enhance the consent process, firmly anchoring improvements within the Belmont principles of Respect for Persons, Beneficence, and Justice [14].
Current initiatives, including recent calls to action from regulatory bodies like the US Food and Drug Administration (FDA), emphasize a collaborative approach to clarifying and improving informed consent for clinical trial participants [59] [60]. This involves creating consent documents and processes that are not only legally compliant but also clear, understandable, and genuinely informative, thereby fulfilling the research community's fundamental ethical obligation [60].
Foundational Ethical Principles
The Belmont Report establishes three core ethical principles that must guide all human subjects research. The informed consent process is the primary vehicle for translating these principles into practice.
The Belmont Report and Informed Consent
Table: Applying Belmont Report Principles to Informed Consent
| Belmont Principle | Definition | Application to Informed Consent |
|---|---|---|
| Respect for Persons | Recognition of the autonomy of individuals and the need to protect those with diminished autonomy [14]. | Requires a meaningful consent process where prospective participants are provided with all relevant information and can comprehend it to make a voluntary decision [14]. |
| Beneficence | The obligation to maximize possible benefits and minimize possible harms [14]. | Mandates the disclosure of anticipated risks and benefits of research as part of the consent process, allowing individuals to assess potential impacts on their well-being [14]. |
| Justice | The requirement to ensure the equitable distribution of the risks and benefits of research [14]. | Addresses the need for equitable selection of subjects and ensuring that the populations who bear the risks of research are those most likely to benefit from its outcomes [14]. |
The requirements for Respect for Persons are satisfied when subjects are provided with a meaningful consent process in which they are given all relevant information about a study that a reasonable person would need and can fully comprehend the information provided [14]. This principle is operationalized through a robust, participant-centric informed consent process.
It is crucial to recognize that these principles can, at times, conflict with one another. For example, considerations of Beneficence must be balanced against the obligation to respect subject autonomy (Respect for Persons) and the demands of Justice [14]. Institutional Review Boards (IRBs) and researchers must consider the particulars of each study and its participant population to find the appropriate balance [14].
Current Challenges and Evolving Contexts
Despite its ethical and regulatory importance, the practical implementation of informed consent faces significant challenges that can impede comprehension and voluntariness.
Procedural and Regulatory Hurdles
In multi-site clinical trials, the informed consent form (ICF) process often becomes a maze of negotiations, leading to delays that postpone trial initiation. Key hurdles include [61]:
- Multiple Handoffs: Each entity—sponsor, Contract Research Organization (CRO), site, and IRB—reviews and modifies the document, creating a cycle of back-and-forth revisions even after a central IRB has completed its review.
- Conflicting Language: Disputes frequently arise over state-specific legal requirements (e.g., Illinois' Genetic Information Privacy Act) or institutional policies, with different sites sometimes interpreting the same law differently.
- Resource Constraints: Many institutions lack dedicated legal counsel for research, forcing study teams to manage both legal and operational reviews, adding days or weeks to the process [61].
The Impact of Emerging Technologies and Datafication
The proliferation of new data types and technologies presents novel ethical challenges that traditional consent models must evolve to address. Social science research, which increasingly deals with digital data, algorithms, and artificial intelligence, is experiencing a shift from rigid procedural ethics to more discipline-specific, methods-based ethical principles [62]. This evolution highlights a broader need for ethical frameworks that can adapt to new methodologies.
In this "datafied" environment, ethics training must move beyond a narrow focus on securing informed consent to enable a critical understanding of the techno-centric environment and the power dynamics embedded in technology and data [63]. This approach, often called critical data literacy, is defined as "the ability to critically engage with datafication by reflecting on the societal implications of data processing and implementing this understanding in practice" [63]. For consent to be meaningful in studies involving complex data, participants need to understand not just the initial data collection, but also potential future uses and the implications of combining data from different sources, which can remove anonymity or be used to predict behavior [63].
Methodologies for Optimization and Assessment
Optimizing the consent process requires a methodological approach grounded in evidence and best practices. The following sections outline proven strategies and experimental protocols for assessment and improvement.
Streamlining and Harmonization Strategies
To overcome procedural hurdles, industry experts recommend several practical solutions [61]:
- Negotiate Early: Pre-negotiating institution-specific language during the Clinical Trial Agreement (CTA) phase can prevent conflicts during later IRB review. Aligning on language early ensures smoother approvals.
- Pre-Vetting Templates: Institutions that proactively work with sponsors to standardize consent language see significant reductions in review cycles. Pre-approved templates address common issues upfront.
- Use Ancillary Documents: Site-specific details (e.g., parking information, financial office contacts) can be communicated through separate participant-facing materials rather than being embedded in the main consent form. This keeps the ICF streamlined and easier to review.
Experimental Protocol: Multi-Analyst Approach to Consent Language Robustness
A multi-analyst study can be employed to assess the robustness and comprehensibility of consent forms and processes. This methodology engages multiple independent analysts to evaluate the same consent materials, examining how different analytical choices affect the assessment of readability, risk communication, and overall clarity.
Table: Key Phases of a Multi-Analyst Study for Consent Evaluation
| Phase | Description | Key Actions |
|---|---|---|
| 1. Recruiting Co-Analysts | Engage a diverse group of analysts with relevant expertise (e.g., bioethicists, clinical researchers, legal experts, patient advocates). | Define expertise criteria; aim for a minimum of 3-5 analysts to identify meaningful variability [64]. |
| 2. Providing Materials & Tasks | Provide the consent form and a clear set of research questions. | Tasks may include evaluating the readability score, identifying key risks, assessing the clarity of procedures, and judging voluntariness of consent [64]. |
| 3. Independent Analysis | Each analyst independently applies their own expertise and methods to evaluate the materials. | Analysts document their chosen assessment criteria, methodology, and findings without collaboration [64]. |
| 4. Processing Results | Compare and synthesize the findings from all analysts. | Identify where assessments converge (indicating robustness) or diverge (indicating areas sensitive to interpretation). Analyze reasons for discrepancies [64]. |
| 5. Reporting | Document the methodology, variability in assessments, and consensus findings. | Transparently report the range of analytical approaches used and how they influenced the evaluation of the consent process [64]. |
This approach allows researchers to identify aspects of the consent form that are consistently understood versus those where interpretation varies significantly, providing a data-driven basis for refinement. When results from independent analysts converge, greater confidence in the quality of the consent materials is warranted. When they diverge, it signals a need to clarify those specific elements [64].
Table: Essential Tools for Developing and Optimizing Informed Consent
| Tool or Resource | Function | Application in Consent Process |
|---|---|---|
| Pre-Vetted Consent Templates | Standardized, pre-approved language for common trial elements. | Streamlines IRB review, reduces back-and-forth negotiations, and ensures regulatory compliance across sites [61]. |
| Readability Analyzers | Software tools that calculate quantitative readability scores (e.g., Flesch-Kincaid). | Provides an objective measure of the language complexity of a consent form, helping to meet the requirement for "understandable" language [61]. |
| Ancillary Information Sheets | Separate documents for site-specific or non-core study information. | Keeps the main consent form focused on essential risks and procedures, improving comprehension of critical information [61]. |
| Digital Consent Platforms | Interactive digital systems for presenting and obtaining consent. | Can enhance understanding through embedded multimedia (videos, quizzes) and provide a clear audit trail. |
| Multi-Analyst Study Framework | A structured methodology for evaluating research materials using multiple independent analysts. | Assesses the robustness and interpretability of consent forms by identifying areas where understanding may vary [64]. |
An Integrated Workflow for Ethical Consent
The following diagram illustrates a logical workflow for developing and implementing an optimized consent process that is grounded in Belmont principles and incorporates the strategies and assessments discussed in this guide.
Optimizing the informed consent process is an ongoing ethical imperative, not a one-time regulatory hurdle. By systematically integrating the foundational principles of the Belmont Report with practical strategies—such as early negotiation of consent language, the use of pre-vetted templates, and rigorous assessment methods like the multi-analyst approach—researchers can significantly enhance participant comprehension and voluntariness. Furthermore, acknowledging the evolving challenges posed by new data technologies and adopting a critical data literacy perspective ensures that the consent process remains robust and meaningful in a changing research landscape. A collaborative and principled focus on consent is essential for upholding the integrity of clinical research and maintaining the trust of the individuals who make this research possible.
Risk-Benefit Analysis in High-Risk, High-Reward Research (e.g., Gene Therapy)
The pursuit of groundbreaking medical treatments, such as gene therapies for inherited retinal diseases or cancers, represents a paradigm shift in medicine, offering potential cures for previously untreatable conditions [65] [66]. However, these innovative approaches carry substantial risks, including potential allergic reactions, damage to organs, or even certain types of cancer [65]. Conducting a rigorous risk-benefit analysis for such research is not merely a regulatory formality but a fundamental ethical obligation. This obligation is rooted in the Belmont Report's foundational principles—Respect for Persons, Beneficence, and Justice—which provide the ethical framework for all human subjects research in the United States [1] [3]. This whitepaper provides a technical guide for researchers and drug development professionals, detailing how to implement a quantitative risk-benefit analysis structured by these principles, ensuring that scientific ambition is consistently aligned with ethical responsibility.
The Belmont Report's Ethical Framework
The Belmont Report, published in 1979, establishes three core ethical principles that must guide human subjects research. These principles are not abstract ideas but must be operationalized through specific applications [1] [3].
Core Ethical Principles
- Respect for Persons: This principle acknowledges the autonomy of individuals and requires protecting those with diminished autonomy. It is implemented through the process of informed consent, which requires that subjects enter research voluntarily and with adequate information presented in an understandable way. This includes detailing the research procedures, their purposes, potential risks and benefits, and alternative options, alongside a statement that the subject can withdraw at any time [1].
- Beneficence: This principle extends beyond simply "do no harm" to an affirmative obligation to "maximize possible benefits and minimize possible harms" [1]. For gene therapy research, this translates into a rigorous and systematic assessment of risks and benefits. The Belmont Report emphasizes that the risks must be justified by the anticipated benefits, either to the subject or to society [1].
- Justice: The principle of justice requires the fair selection of subjects, ensuring that the burdens and benefits of research are distributed equitably. Researchers must avoid selecting subjects simply because of their easy availability, compromised position, or due to societal biases [1]. This is particularly relevant in gene therapy, where access to potentially curative treatments must be considered fairly.
The following diagram illustrates the relationship between these principles and their practical applications in the research workflow:
Quantitative Risk Analysis Frameworks and Methods
A robust risk-benefit analysis must move beyond qualitative assessments to a data-driven, quantitative approach. This allows for objective decision-making, prioritization of risks, and enhanced communication with stakeholders [67] [68] [69].
Foundational Concepts
Quantitative risk analysis uses numerical values and statistical models to understand financial and operational uncertainty [67] [69]. It involves:
- Data Collection and Validation: Gathering reliable historical data on risks, market trends, and operational metrics [67] [68].
- Risk Identification and Measurement: Assigning numerical values to the probability of a risk occurring and the potential impact if it does [68].
- Statistical Modeling and Simulation: Using mathematical models to predict potential outcomes under different conditions [67].
Common Frameworks and Their Applications
The table below summarizes several prominent quantitative risk frameworks applicable to gene therapy research.
Table 1: Quantitative Risk Analysis Frameworks
| Framework | Core Function | Primary Application in Research |
|---|---|---|
| Monte Carlo Simulation | Uses repeated random sampling to simulate a range of possible outcomes based on identified risk variables [68]. | Modeling clinical trial outcomes, forecasting manufacturing success rates, and predicting patient recruitment timelines. |
| Value at Risk (VaR) | Estimates the maximum potential financial loss over a specific time period with a given confidence level (e.g., 95%) [68]. | Quantifying the financial exposure of a research portfolio to market shifts or failed clinical trials. |
| Expected Shortfall (ES) | Measures the average loss in scenarios that exceed the VaR threshold, providing more insight into extreme "tail risks" [68]. | Assessing the impact of worst-case scenarios, such as a severe adverse event leading to trial suspension. |
| Credit Risk Models | Assess the likelihood that a borrower or counterparty will default on an obligation [68]. | Evaluating the financial stability of partners, such as contract research organizations (CROs) or manufacturing partners. |
| Factor Analysis of Information Risk (FAIR) | A model for understanding, analyzing, and quantifying operational risk [69]. | Quantifying cybersecurity and data integrity risks associated with handling sensitive genetic and patient data. |
The process for implementing these frameworks is methodical, as shown in the workflow below:
Application to Gene Therapy Clinical Trials
Gene therapy research presents a compelling use case for applying these structured risk-benefit analyses, given its high potential reward and significant, complex risks.
Documented Benefits and Risks
The benefits of genetic therapies are substantial, including the potential to prevent, treat, or cure inherited disorders like cystic fibrosis, hemophilia, and sickle cell disease, as well as certain cancers [65]. As of 2022, the FDA has approved gene therapies for conditions like Leber congenital amaurosis and specific blood cancers [65].
However, potential risks are equally significant and must be quantified. These can include certain types of cancer, allergic reactions, or damage to organs or tissues from the injection procedure itself [65]. Furthermore, the NIH does not fund research involving genome editing in human sperm, eggs, or embryos, as these germline changes would be heritable and could have "unanticipated effects" [65].
Quantitative Analysis of Patient Eligibility and Outcomes
A critical component of risk-benefit analysis is setting realistic expectations for patient eligibility and outcomes. For instance, for the approved therapy voretigene neparvovec-rzyl (Luxturna), eligibility is strictly limited to patients with "confirmed biallelic RPE65 mutation–associated retinal dystrophy" [66]. This means patients with only one variant, or with variants of uncertain significance, are not eligible. A 2024 international survey revealed a significant gap in understanding, with 53% of patients and caregivers believing gene therapy could restore vision to normal, underscoring the need for clear, data-driven communication about variable outcomes, which may only slow disease progression or improve mobility in dim light, rather than fully restore vision [66].
Table 2: Gene Therapy Risk-Benefit Analysis: Key Quantitative & Qualitative Factors
| Analysis Dimension | Key Considerations | Data Source & Measurement Approach |
|---|---|---|
| Eligibility & Access | - Genetic criteria (e.g., biallelic mutations) [66].- Presence of viable retinal cells [66].- Limited treatment centers (e.g., 19 for Luxturna in the US as of 2025) [66]. | - Genetic test results.- Ocular imaging and functional tests.- Geographic and logistical mapping. |
| Clinical Outcomes | - Goal is often to slow progression, not cure [66].- Potential for unexpected adverse events (e.g., post-approval retinal atrophy) [66].- Impact on eligibility for future therapies [66]. | - Clinical trial endpoints (e.g., mobility test scores).- Long-term safety monitoring registries.- Patient-reported outcome measures. |
| Financial Risks | - High cost of therapy, drug, and associated care [66].- Insurance coverage variability [66].- Indirect costs (travel, time off work) [66]. | - Cost-effectiveness models (e.g., QALYs).- Insurance claim analysis.- Patient cost-survey data. |
The Scientist's Toolkit: Essential Reagents and Materials
The following table details key reagents and materials essential for conducting gene therapy research and clinical trials, with an explanation of their critical function in the process.
Table 3: Research Reagent Solutions for Gene Therapy
| Reagent / Material | Critical Function in Research & Development |
|---|---|
| Viral Vectors (e.g., AAV) | The primary delivery vehicle for transporting therapeutic genetic material into target human cells. Different serotypes have different tropisms, dictating which tissues they can effectively target [65] [66]. |
| Therapeutic Transgene Cassette | The functional genetic unit containing the correct version of the gene being replaced or supplemented, along with necessary regulatory sequences (promoters, enhancers) to control its expression [66]. |
| Cell Lines (e.g., HEK293) | Used as a factory for producing the viral vectors at the scale and purity required for clinical use. These are often immortalized lines optimized for high-yield vector production. |
| Purification Chromatography | Essential for downstream processing to separate and purify the viral vectors from cell culture contaminants, ensuring final product safety and potency. |
| qPCR/ddPCR Assays | Used to quantify the concentration (titer) of viral vectors throughout the manufacturing process and to assess the vector genome copy number in transduced cells, a key pharmacokinetic metric. |
| Immunoassay Kits (e.g., ELISA) | Critical for detecting and quantifying immune responses against the viral vector capsid or the newly expressed transgene product, which is a major risk factor for efficacy and safety [65]. |
In high-risk, high-reward research domains like gene therapy, a rigorous, quantitative risk-benefit analysis is the indispensable bridge between revolutionary scientific potential and responsible ethical practice. By integrating the foundational ethical principles of the Belmont Report—Respect for Persons, Beneficence, and Justice—with robust quantitative frameworks like Monte Carlo simulations and Value at Risk models, researchers and drug development professionals can navigate this complex landscape with greater clarity and confidence. This structured approach enables objective decision-making, improves stakeholder communication, and ensures that the pursuit of medical innovation remains firmly grounded in the protection of human subjects and the equitable distribution of both the burdens and benefits of research.
Addressing Institutional and Systemic Barriers to Ethical Research Practices
The foundational principles of the Belmont Report—Respect for Persons, Beneficence, and Justice—provide the ethical bedrock for human subjects research [1]. Despite widespread adoption of these principles, significant institutional and systemic barriers prevent their consistent implementation across the research ecosystem. The exclusion of historically marginalized populations in research contributes to a negative feedback loop that perpetuates health inequities [70]. This whitepaper examines these barriers through the Belmont framework and provides technical guidance for researchers, scientists, and drug development professionals to identify and address these challenges systematically. Contemporary research reveals that systemic barriers permeate the entire research process, from participant recruitment to data sharing and dissemination, requiring multilevel solutions that embed equity, diversity, and inclusion (EDI) at each stage [70]. By understanding these structural challenges and implementing practical solutions, the research community can work toward a more equitable and ethically sound research paradigm.
The Belmont Framework and Contemporary Research Challenges
Foundational Ethical Principles
The Belmont Report establishes three fundamental ethical principles for human subjects research [1]:
- Respect for Persons: This principle incorporates two ethical convictions: individuals should be treated as autonomous agents, and persons with diminished autonomy are entitled to protection. It requires researchers to ensure subjects enter research voluntarily with adequate information and protect those with reduced autonomy.
- Beneficence: Researchers have an obligation to secure the well-being of participants through two complementary rules: do not harm, and maximize possible benefits while minimizing possible harms.
- Justice: This principle requires fair selection of subjects and equitable distribution of research risks and benefits across society, preventing the systematic selection of subjects based on easy availability, compromised position, or societal biases.
Modern Research Context and Ethical Gaps
While these principles provide ethical guidance, their implementation faces contemporary challenges. Research indicates that marginalized communities—including racialized and Black individuals, 2SLGBTQIA+ communities, Indigenous peoples, women and girls, and individuals with disabilities—continue to experience systemic under-recruitment and tokenistic participation in research [70]. This exclusion violates the justice principle of Belmont by distributing research burdens and benefits inequitably. The research ecosystem often contains entrenched power imbalances, insufficient cultural safety, and widespread discrimination that prevent the consistent application of ethical principles [70]. These challenges are particularly acute in child health research, where strict ethical guidelines designed to protect children may inadvertently create additional barriers to inclusive participation [70].
Table 1: Quantitative Analysis of Systemic Barriers in Child Health Research (2020-2022)
| Marginalized Community | Number of Publications Identifying Barriers | Primary Barrier Types |
|---|---|---|
| Racialized Individuals | 30 | Misleading conceptualizations of race, discrimination, power dynamics |
| Black Individuals | 20 | Mistrust, homogeneous recruitment strategies, cultural barriers |
| Women and Girls | 10 | Patriarchal structures, underrepresentation in research design |
| Indigenous Peoples | 9 | Colonial research practices, lack of cultural safety |
| Children with Disabilities | 7 | Accessibility barriers, ableist assumptions |
| 2SLGBTQIA+ Individuals | 4 | Stigma, binary conceptualizations of gender/sexuality |
Source: Adapted from scoping review of 53 publications on EDI in child health research [70]
Systemic Barriers in the Research Ecosystem
Institutional and Organizational Barriers
Research institutions often create environments where a mismatch exists between what tool creators know is necessary for ethical, actionable science and what is possible given institutional constraints [71]. These institutional barriers include:
- Reward Structures: Academic promotion and tenure committees often prioritize publication quantity and journal prestige over ethical considerations, community engagement, or inclusive research practices.
- Resource Allocation: Research funding mechanisms frequently fail to allocate sufficient resources for meaningful community engagement, culturally safe practices, or additional supports required for inclusive research with marginalized groups.
- Administrative Bureaucracy: Institutional review processes may prioritize risk mitigation over ethical engagement, creating cumbersome procedures that deter community participation or innovative methodological approaches.
- Data Sharing Infrastructure: Research institutions often lack the technical infrastructure and support systems needed for ethical data sharing, with 34% of researchers reporting they "usually" or "always" lack sufficient time to prepare data for sharing, and 27% noting they don't have rights to share data [72].
Methodological and Procedural Barriers
At the procedural level, researchers face significant challenges in implementing ethically sound protocols:
- Protocol Documentation: Experimental protocols often lack sufficient detail for replication or fail to address ethical considerations adequately. Analysis reveals that fewer than 20% of highly-cited publications have adequate descriptions of study design and analytic methods [73].
- Resource Identification: Over 54% of biomedical research resources are not uniquely identifiable in the literature, creating reproducibility challenges and ethical concerns about proper attribution [73].
- Recruitment Limitations: Barriers include logistical challenges, limited access to research opportunities, homogeneous strategies, financial constraints, language and cultural barriers, stigma, mistrust, and concerns about adverse effects [70].
Experimental Protocols for Ethical Research Practice
Comprehensive Protocol Development Framework
Robust experimental protocols serve as both methodological and ethical guides for research. A comprehensive protocol should function like a detailed recipe—sufficiently thorough that a trustworthy non-lab-member psychologist could run it correctly from the script alone [74]. Analysis of over 500 published and unpublished protocols reveals that effective ethical protocols include 17 fundamental data elements that ensure both reproducibility and ethical practice [73]. These protocols must be tested and refined before implementation, including self-testing, lab member review, and supervised runs with naive participants [74].
Table 2: Essential Data Elements for Ethical Experimental Protocols
| Category | Data Elements | Ethical Considerations |
|---|---|---|
| Study Setup | Research question, Hypothesis, Experimental units | Alignment with Belmont principles, Benefit-risk analysis |
| Materials | Reagents & equipment (with unique identifiers), Preparation methods | Proper attribution, Quality control for participant safety |
| Participants | Inclusion/exclusion criteria, Recruitment methods, Sample size | Justice in participant selection, Respect for autonomy |
| Procedure | Step-by-step instructions, Timing, Controls, Randomization | Beneficence through harm minimization, Consistency |
| Data Management | Data collection methods, Quality control, Analysis plan | Respect through privacy protection, Transparency |
| Ethical Safeguards | Informed consent process, Debriefing materials, Conflict of interest disclosure | Respect for persons, Accountability |
Source: Adapted from guideline for reporting experimental protocols in life sciences [73]
Specialized Methodologies for Inclusive Research
Implementing the Belmont principles requires specialized methodologies that address the unique needs of diverse populations:
- Cultural Safety Protocols: Research teams should develop specific protocols for creating culturally safe environments, including appropriate greeting and consent processes that acknowledge power imbalances and historical trauma [70] [74].
- Participant Engagement Methods: Ethical research requires meaningful engagement throughout the research process, not merely as subjects but as partners in research design, implementation, and dissemination.
- Accessibility Adaptations: Research procedures must be adapted to accommodate participants with disabilities, including physical accessibility, cognitive accessibility, and communication accommodations.
- Data Sovereignty Practices: Particularly when working with Indigenous communities, protocols must respect data sovereignty principles and community ownership of information.
Table 3: Research Reagent Solutions for Ethical Research Practice
| Tool/Resource | Function | Ethical Application |
|---|---|---|
| SMART Protocols Ontology | Standardized representation of experimental protocols | Ensures comprehensive reporting of methods and materials for reproducibility and ethical accountability [73] |
| Resource Identification Portal | Universal identification of research resources (antibodies, plasmids, etc.) | Enables proper attribution and replication, supporting beneficence through research quality [73] |
| Cultural Safety Assessment Tool | Framework for evaluating cultural appropriateness of research practices | Addresses respect for persons through culturally safe engagement methods [70] |
| Community Advisory Committee | Structured community input throughout research process | Ensures justice through equitable community representation and power-sharing [70] |
| Data Sharing Infrastructure | Technical platforms for ethical data sharing | Facilitates transparency while respecting privacy and data sovereignty [72] |
| Ethical Contrast Assessment | Tools for ensuring accessibility in visual materials | Supports justice through accessible research dissemination [75] |
Implementation Framework and Pathway Forward
Multilevel Intervention Strategy
Addressing systemic barriers requires coordinated interventions across multiple levels of the research ecosystem. The socio-ecological model provides a framework for systematically mapping barriers and developing tailored, multilevel solutions [70]. This approach recognizes that individual behavior change alone is insufficient without corresponding institutional and policy reforms.
Metrics for Evaluating Ethical Research Practice
Establishing comprehensive metrics is essential for assessing progress in addressing systemic barriers:
- Participant Diversity Tracking: Quantitative monitoring of recruitment and retention across demographic groups to ensure justice in participant selection.
- Community Engagement Quality Assessment: Structured evaluation of the depth and quality of community partnerships throughout the research process.
- Protocol Comprehensiveness Scoring: Standardized assessment of experimental protocols against the 17 essential data elements for ethical research [73].
- Data Sharing Compliance Rates: Monitoring adherence to ethical data sharing requirements and identification of persistent barriers [72].
The Belmont Report's principles of respect for persons, beneficence, and justice remain profoundly relevant but inadequately realized in contemporary research ecosystems. Addressing the institutional and systemic barriers to ethical research practices requires deliberate, multilevel strategies that transform both the structures and cultures of research. By implementing comprehensive experimental protocols, developing authentic community partnerships, restructuring reward systems, and embedding equity throughout the research lifecycle, the scientific community can bridge the gap between ethical aspiration and practice. This transformation is both a moral imperative and a scientific necessity—producing research that is not only more ethical but also more rigorous, reproducible, and impactful. The technical guidance provided in this whitepaper offers a pathway toward realizing this transformative vision, ensuring that research fulfills its potential to benefit all communities equally.
The Belmont Report's Enduring Legacy: Impact on Regulations and Comparison with Other Ethical Frameworks
This technical guide examines the foundational role of the Belmont Report in shaping the Federal Policy for the Protection of Human Subjects, known as the Common Rule (45 CFR 46). We analyze how the Report's three ethical principles—Respect for Persons, Beneficence, and Justice—were systematically translated into regulatory requirements governing federally funded research. The analysis includes the historical context of the Report's creation, its direct influence on the Common Rule's structure, and subsequent regulatory updates. Designed for researchers, scientists, and drug development professionals, this whitepaper provides a framework for understanding the ethical underpinnings of contemporary human research protections.
The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was created by the National Research Act of 1974 in direct response to ethical transgressions in research, most notably the Tuskegee Study of Untreated Syphilis in the Negro Male [76] [6]. This study, which continued for 40 years, failed to inform participants of their diagnosis, withheld treatment, and did not offer participants the opportunity to withdraw [76]. The ensuing public outrage highlighted the critical need for a comprehensive federal policy to protect human subjects.
The National Commission's mandate was to identify the comprehensive ethical principles that should guide research involving human subjects. In 1978, the Commission delivered the Belmont Report, named after the conference center where it was drafted [1]. The report was formally published in the Federal Register in April 1979 [3]. Its primary contribution was the articulation of three core ethical principles intended to serve as an analytical framework for resolving ethical problems in research [2]. The Common Rule (45 CFR 46), formally adopted in 1991, operationalizes these principles into enforceable regulations [76].
The Ethical Framework of the Belmont Report
The Belmont Report establishes three fundamental ethical principles that form the moral foundation for human subjects research in the United States. The principles are not merely a checklist but provide a compass for ethical decision-making [2].
The Principle of Respect for Persons
This principle incorporates two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection [1] [6]. The requirement to acknowledge autonomy translates into the moral and regulatory requirement for voluntary informed consent. Prospective subjects must be given adequate information in an understandable format, must be allowed to ask questions, and must be free from coercion or undue influence [1] [6]. The Report specifically recommends that consent information include the research procedure, purposes, risks and anticipated benefits, and alternative procedures [1]. The second conviction requires that special protections be afforded to vulnerable populations, such as children, prisoners, and individuals with cognitive impairments, with the extent of protection based on the risk of harm and likelihood of benefit [1].
The Principle of Beneficence
This principle extends beyond the simple injunction to "do no harm" to an affirmative obligation to secure the well-being of research participants [1]. The principle of beneficence is expressed through two complementary rules: "(1) do not harm and (2) maximize possible benefits and minimize possible harms" [1] [6]. In practice, this requires researchers and Institutional Review Boards (IRBs) to conduct a systematic assessment of risks and benefits [1] [2]. The Belmont Report outlines a method for IRBs to rigorously gather and assess all aspects of the research to determine if the risks to subjects are justified by the anticipated benefits, ensuring the process is non-arbitrary and fact-based [1].
The Principle of Justice
The principle of justice addresses the fair distribution of the burdens and benefits of research [2]. It requires that the selection of research subjects be scrutinized to avoid systematically selecting populations simply because of their easy availability, compromised position, or social, racial, sexual, or economic status [1] [6]. Historically, vulnerable and disadvantaged populations often bore the burdens of research while more privileged populations enjoyed its benefits. The principle of justice demands that no single group—whether defined by age, race, class, or gender—should disproportionately bear the risks or disproportionately reap the benefits of research participation [2].
Table 1: Core Ethical Principles of the Belmont Report and Their Moral Requirements
| Ethical Principle | Core Meaning | Moral Requirements |
|---|---|---|
| Respect for Persons | Recognition of the autonomy of individuals and protection for those with diminished autonomy [1]. | - Obtain voluntary informed consent- Protect vulnerable populations |
| Beneficence | Obligation to secure the well-being of participants by maximizing benefits and minimizing harms [1]. | - Conduct systematic risk/benefit assessment- Do not harm |
| Justice | Fairness in the distribution of the burdens and benefits of research [2]. | - Ensure equitable selection of subjects- Avoid exploitation of vulnerable populations |
Operationalizing Ethics: The Common Rule (45 CFR 46)
The Common Rule (45 CFR 46) is the federal regulation that directly translates the ethical principles of the Belmont Report into specific, enforceable requirements for research conducted or supported by federal departments and agencies [77] [76].
From Respect for Persons to Informed Consent
The principle of Respect for Persons is most directly implemented through the Common Rule's detailed informed consent requirements [76]. The regulations mandate that informed consent must contain specific elements to ensure participants are adequately informed. These elements include [76]:
- A statement that the study involves research, an explanation of its purposes, and the expected duration of participation.
- A description of any foreseeable risks or discomforts.
- A description of any benefits to the subject or others.
- Appropriate alternative procedures or courses of treatment.
- A statement describing the extent of confidentiality of records.
- For research involving more than minimal risk, an explanation of compensation and treatment available for research-related injury.
- The crucial statement that participation is voluntary, refusal carries no penalty, and the subject may discontinue at any time.
The 2019 revisions to the Common Rule further strengthened this process by requiring that consent forms begin with a "concise and focused presentation of the key information" to help prospective subjects understand the reasons for or against participation, improving comprehension of complex forms [76] [78].
From Beneficence to IRB Review and Risk-Benefit Analysis
The principle of Beneficence is embedded in the Common Rule's requirement for independent review by an Institutional Review Board (IRB) [76] [6]. Before a study can begin, researchers must submit a proposal to the IRB, which is charged with ensuring the protection of human subjects. A key function of the IRB is to assess the ratio of risks to benefits in the proposed research, directly applying the Belmont Report's principle of beneficence [6]. The IRB must determine that risks are minimized and are reasonable in relation to anticipated benefits [76]. The revised Common Rule also eliminated continuing review for some minimal-risk studies, such as those involving only data analysis, reflecting a more nuanced application of beneficence that reduces administrative burden without compromising safety [78].
From Justice to Equitable Subject Selection
The Common Rule upholds the principle of Justice through provisions that require equitable selection of subjects [6]. IRBs are required to scrutinize the proposed subject population to ensure that no group is selected merely for its easy availability, vulnerability, or compromised legal status [6]. The regulations also include additional protections for vulnerable populations, such as pregnant women, prisoners, and children (outlined in Subparts B, C, and D of 45 CFR 46), ensuring that these groups are not unjustly targeted for research bearing disproportionate risks [6].
Table 2: Translating Belmont Report Principles into Common Rule Requirements
| Belmont Principle | Regulatory Application in Common Rule | Key Regulatory Citations |
|---|---|---|
| Respect for Persons | - Requirements for informed consent (§46.116) [76]- Additional protections for vulnerable populations (Subparts B, C, D) [6] | 45 CFR 46.116 |
| Beneficence | - IRB review and approval (§46.109) [6]- IRB assessment of risks and benefits [6]- Criteria for IRB approval (§46.111) | 45 CFR 46.109, 45 CFR 46.111 |
| Justice | - IRB review of subject selection (§46.111) [6]- Equitable distribution of risks and benefits [2] | 45 CFR 46.111 |
The Regulatory Evolution: Key Revisions to the Common Rule
The Common Rule was updated in 2017, with most provisions becoming effective in 2019, marking its first major revision in decades [76]. These changes further refine the application of the Belmont principles.
Enhanced Informed Consent (Respect for Persons)
A significant revision was the introduction of the "Key Information" requirement. Consent forms must now begin with a concise and focused presentation of the most important information to help prospective subjects make an informed decision [76]. This change addresses the challenge that lengthy, complex forms can obscure critical details, thereby better respecting participant autonomy.
Regulations for Biospecimens and Data (Beneficence & Respect)
The revised rule added new elements for consent concerning identifiable private information and identifiable biospecimens [76]. Subjects must be informed if their biospecimens might be used for commercial profit, if the research includes whole genome sequencing, and whether clinically relevant research results will be disclosed to them [76]. These provisions enhance beneficence by ensuring participants understand potential future uses of their biological materials and respect for persons by empowering them with knowledge about the return of significant findings.
Streamlined Review Processes (Beneficence)
The revised Common Rule promotes efficient application of resources by eliminating continuing review for many minimal-risk studies, such as those eligible for expedited review or that have progressed to the point of involving only data analysis [78]. This allows IRBs to focus oversight on higher-risk studies, a practical application of beneficence that benefits the research system as a whole.
The Researcher's Toolkit: Implementing Ethical Review
For researchers and drug development professionals, understanding the pathway from ethical principle to regulatory compliance is a critical competency. The following workflow and toolkit outline the key components for designing research that aligns with both the spirit and the letter of the regulations.
Table 3: Essential Documentation for Ethical Research Compliance
| Document or Tool | Primary Function | Connection to Belmont Principles |
|---|---|---|
| IRB Research Protocol | Detailed description of research design, methods, and procedures submitted for review. | Serves as the primary document for IRB assessment of Beneficence (risks/benefits) and Justice (subject selection). |
| Informed Consent Form (ICF) | Legal and ethical document providing key information to potential participants. | Directly implements Respect for Persons by ensuring voluntary participation based on comprehension. |
| Key Information Section | New, concise summary at the beginning of the ICF. | Enhances Respect for Persons by facilitating understanding of core study elements. |
| Recruitment Materials | Advertisements, scripts, and flyers used to enroll subjects. | Reviewed by IRB to ensure Justice (equitable selection) and avoid coercion (Respect for Persons). |
| Data Safety Monitoring Plan | Procedures for protecting participant data and monitoring study safety. | Upholds Beneficence (minimizing harm) and Respect for Persons (protecting confidentiality). |
The Belmont Report is not a historical relic but a living ethical framework whose principles are directly encoded into the Code of Federal Regulations. For researchers, scientists, and drug development professionals, understanding that the Common Rule's requirements for informed consent, IRB review, and equitable subject selection are practical applications of Respect for Persons, Beneficence, and Justice is fundamental. This understanding transforms regulatory compliance from a bureaucratic exercise into a meaningful practice of research ethics. As the Common Rule continues to evolve, as evidenced by the 2019 updates, its development remains guided by the foundational compass provided by the Belmont Report, ensuring that the protection of human subjects remains the cornerstone of ethical research.
The Belmont Report, published in 1979, established a foundational ethical framework for research involving human subjects through its three core principles: Respect for Persons, Beneficence, and Justice [1]. This whitepaper examines how these principles have directly shaped the regulatory oversight and policy development for gene therapy in the decades since the report's publication. As gene therapy has evolved from a theoretical concept to a clinical reality with over 700 active investigational new drug applications currently with the FDA [79], the Belmont framework has provided the ethical bedrock for navigating unique challenges in safety assessment, informed consent, and equitable access. We analyze the translation of ethical principles into concrete regulatory requirements, detail specific experimental methodologies for ethical review, and visualize the integrated oversight system that governs modern gene therapy development.
The Belmont Report was created by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research in response to historical ethical violations in human subjects research, most notably the Tuskegee Syphilis Study [7]. Its publication established three fundamental ethical principles that would become the cornerstone of human subjects protection in the United States:
- Respect for Persons: Acknowledging individual autonomy and protecting those with diminished autonomy [1]
- Beneficence: The obligation to maximize benefits and minimize potential harms [1]
- Justice: Ensuring fair distribution of both the burdens and benefits of research [1]
These principles were subsequently codified into federal regulations through the Common Rule (45 CFR part 46), which outlines duties for institutional review boards (IRBs) and researchers [7]. For the field of gene therapy, which involves modifying or manipulating gene expression in living cells for therapeutic use [80], these principles have provided an essential framework for addressing the unique ethical challenges posed by these innovative biologics.
Historical Context and Regulatory Evolution
From Belmont to Gene Therapy Oversight
The development of gene therapy oversight reflects a direct application of Belmont principles to emerging technologies. The Recombinant DNA Advisory Committee (RAC) was established at the NIH in 1974 to address the ethical, legal, and social implications of emerging genetic technologies [79]. The tragic 1999 death of Jesse Gelsinger during a gene therapy trial brought closer scrutiny of the field, leading to "increased regulatory oversight" and enhanced safety precautions [79], embodying the Belmont principle of Beneficence.
Table: Major Milestones in Gene Therapy Oversight
| Year | Event | Significance |
|---|---|---|
| 1974 | Establishment of RAC | Created advisory body for emerging genetic technologies |
| 1979 | Publication of Belmont Report | Established three core ethical principles for human subjects research |
| 1990 | First U.S. human gene therapy trial | FDA oversight of pediatric patients with adenosine deaminase deficiency [79] |
| 1999 | Death of Jesse Gelsinger | Led to enhanced safety monitoring and transparency requirements [79] |
| 2017 | First FDA-approved gene therapies | Marked transition from experimental to approved treatments [79] |
| 2018 | FDA/NIH oversight streamlining | Reduced duplicative reporting requirements while maintaining ethical safeguards [79] |
Modern Regulatory Framework
The current oversight system for gene therapy represents a maturation of Belmont principles within a streamlined regulatory structure. In 2018, the NIH and FDA proposed efforts to "reduce the duplicative oversight burden" by limiting the role of the RAC in assessing gene therapy protocols, while maintaining robust ethical review through existing clinical research oversight systems [79]. This evolution reflects the field's transition from an emerging, unpredictable technology to one whose risks are better understood and managed within established regulatory frameworks.
The FDA's Center for Biologics Evaluation and Research (CBER) regulates cellular therapy products and human gene therapy products under both the Public Health Service Act and the Federal Food, Drug and Cosmetic Act [80]. The Office of Therapeutic Products (OTP) has replaced the previous Office of Tissues and Advanced Therapies (OTAT), creating a "super office" with increased review capabilities and expertise in cell and gene therapy products [81].
Application of Belmont Principles to Gene Therapy
Respect for Persons in Gene Therapy Informed Consent
The Belmont principle of Respect for Persons requires that individuals be treated as autonomous agents and that those with diminished autonomy receive additional protection [1]. In gene therapy, this translates to comprehensive informed consent processes that address the unique characteristics of these treatments:
- Explanation of permanent nature: Gene therapies often produce permanent changes to the genetic makeup, requiring clear communication about the long-term implications [82]
- Viral vector risks: Consent must cover risks associated with delivery mechanisms, such as immune responses to viral vectors [79]
- Uncertain long-term effects: Given the novelty of many therapies, unknowns about long-term outcomes must be transparently disclosed [79]
- Germ-line implications: Although current therapies target somatic cells, potential effects on reproductive cells must be addressed where relevant [82]
The Belmont Report's requirement for "voluntary consent" with "adequate information" [1] becomes particularly crucial for gene therapies targeting rare diseases, where patient populations may be especially vulnerable to therapeutic misconception.
Beneficence in Risk-Benefit Assessment
The principle of Beneficence requires researchers to "maximize possible benefits and minimize possible harms" [1]. For gene therapy, this necessitates rigorous safety testing and long-term monitoring protocols:
- Preclinical assessment: The FDA's "Preclinical Assessment of Investigational Cellular and Gene Therapy Products" guidance outlines comprehensive safety testing requirements [83]
- Long-term follow-up: Specific guidance on "Long Term Follow-up After Administration of Human Gene Therapy Products" addresses the need for extended safety monitoring [83]
- Risk mitigation strategies: For CRISPR-based therapies, assessment of off-target effects represents a specific safety concern that must be addressed [81] [79]
Table: FDA Guidance Documents Applying Beneficence Principle to Gene Therapy
| Guidance Document | Publication Date | Key Risk-Benefit Considerations |
|---|---|---|
| Human Gene Therapy Products Incorporating Human Genome Editing [83] | 1/2024 | Safety assessment for genome editing technologies |
| Long Term Follow-up After Administration of Human Gene Therapy Products [83] | 1/2020 | Monitoring long-term safety and efficacy |
| Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus [83] | 1/2020 | Specific vector safety testing |
| Preclinical Assessment of Investigational Cellular and Gene Therapy Products [83] | 11/2013 | Comprehensive preclinical safety testing |
Justice in Access and Equity
The Belmont principle of Justice requires fair subject selection and equitable distribution of both research burdens and benefits [1]. This principle faces significant challenges in gene therapy, particularly regarding:
- Ultra-rare diseases: Therapies for conditions affecting very small populations (e.g., "ultra-rare" diseases affecting fewer than 6,000 people) raise development challenges [84]
- Economic barriers: The high upfront costs of gene therapies create access disparities, especially for Medicaid populations [84]
- Selection criteria: Justice requires that subject selection not be based on "easy availability" or "compromised position" [1]
Current policy discussions focus on alternative payment models and coverage requirements to ensure equitable access to gene therapies, particularly for rare diseases that disproportionately affect vulnerable populations [84].
Experimental Protocols for Ethical Review
Protocol for Risk-Benefit Assessment
The Belmont Report outlines a systematic method for IRB members to determine whether research risks are justified by benefits [1]. For gene therapy protocols, this assessment requires specific methodological considerations:
Materials and Reagents:
- Preclinical toxicology data from animal models
- Manufacturing consistency data (CMC information)
- Vector characterization profiles
- Validated potency assays
- Previous clinical experience with similar constructs
Methodology:
- Systematic risk identification: Catalog all potential risks, including vector-related toxicity, off-target effects (for editing approaches), immune responses, and long-term uncertainties [79]
- Benefit analysis: Distinguish between direct therapeutic benefits and knowledge gains; assess probability and magnitude of each [1]
- Alternative assessment: Compare against existing treatment options, including standard care and other investigational approaches [1]
- Risk minimization: Implement monitoring strategies, safety stopping rules, and patient management protocols [83]
- Justification determination: Weigh the net risk-benefit balance, ensuring risks are "reasonable in relation to anticipated benefits" [1]
Protocol for Vulnerability Assessment
The Belmont Report requires special protections for persons with "diminished autonomy" [1]. Gene therapy protocols must include specific assessments when involving potentially vulnerable populations:
Materials:
- Capacity assessment tools
- Independent advocate consultation documentation
- Disease-specific educational materials
- Cultural/linguistic appropriate consent forms
Methodology:
- Vulnerability identification: Determine whether potential subjects have limitations in decision-making capacity due to age, disease state, cognitive impairment, or situational factors [1]
- Proxy consent validation: For subjects unable to consent, ensure proper authorization and alignment with subject's values and interests [1]
- Assent procedures: Develop age-appropriate involvement for pediatric populations [1]
- Undue influence assessment: Evaluate whether disease severity or lack of alternatives compromises voluntary choice [1]
- Monitoring plan: Establish ongoing evaluation of subject understanding and continued willingness to participate [1]
Visualization of Ethical Oversight Framework
The following diagram illustrates how Belmont Report principles are integrated into the gene therapy oversight system, from foundational ethics through regulatory review and ongoing monitoring:
Diagram: Integration of Belmont Report Principles into Gene Therapy Oversight
The Scientist's Toolkit: Research Reagent Solutions
Table: Essential Research Materials for Gene Therapy Development
| Reagent/Material | Function | Ethical Considerations |
|---|---|---|
| Viral Vectors (AAV, Lentivirus) | Gene delivery vehicles | Immune response risks, insertional mutagenesis potential [81] [79] |
| CRISPR-Cas9 Components | Genome editing machinery | Off-target effects, precision of editing [81] [79] |
| CAR-T Cell Constructs | Engineered cellular immunotherapy | Cytokine release syndrome, on-target/off-tumor effects [81] |
| Good Manufacturing Practice (GMP) Grade Materials | Clinical-grade production | Manufacturing consistency, quality control [81] |
| Potency Assays | Product potency measurement | Correlation with clinical effect, reliability [83] |
Current Challenges and Future Directions
Emerging Ethical Challenges
While the Belmont Report provides a durable ethical foundation, gene therapy development faces ongoing challenges in applying its principles:
- Financial feasibility: Developing therapies for "ultra-rare" diseases with limited patient populations presents economic challenges that raise justice concerns [84]
- Manufacturing bottlenecks: Limited production capacity for viral vectors and cell therapies can create access disparities [81]
- Regulatory coordination: Despite streamlining efforts, sponsors still face challenges navigating FDA communications and review processes [81]
- Post-approval monitoring: Long-term follow-up requirements raise practical challenges for assessing durable safety and efficacy [83]
Policy Evolution
Recent policy initiatives aim to address these challenges while maintaining alignment with Belmont principles:
- The START program (Support for clinical Trials Advancing Rare disease Therapeutics) aims to accelerate development for rare diseases with significant unmet need [81]
- Umbrella trial designs allow multiple versions of a therapy to be tested under a master protocol, improving efficiency while maintaining ethical oversight [81]
- Alternative payment models are being explored to address the high upfront costs of gene therapies while ensuring equitable access [84]
The Belmont Report has provided an enduring ethical framework that continues to shape gene therapy oversight and policy decades after its publication. Its three principles—Respect for Persons, Beneficence, and Justice—are clearly reflected in the regulatory guidance, review processes, and policy discussions surrounding modern gene therapy development. As the field evolves with new technologies like CRISPR-based genome editing, the Belmont framework offers a stable foundation for addressing novel ethical challenges while promoting the responsible development of transformative therapies for patients with previously untreatable conditions.
The ethical conduct of research involving human subjects is governed by a series of landmark documents that have evolved in response to historical abuses and emerging ethical challenges. The Belmont Report, published in 1979, represents a pivotal moment in this evolution, distilling ethical principles into a foundational framework that continues to guide researchers, regulators, and institutional review boards today [7]. This analytical whitepaper examines how the Belmont Report's principles serve as the contemporary foundation for ethical research practice when compared with its historical predecessors—the Nuremberg Code (1947) and the Declaration of Helsinki (first adopted in 1964) [85] [86]. For researchers and drug development professionals, understanding this ethical progression is not merely an academic exercise but a practical necessity for designing studies that meet both regulatory requirements and moral imperatives.
The historical context for these documents reveals a steady maturation of research ethics. The Nuremberg Code emerged from the atrocities of Nazi medical experiments, establishing for the first time the absolute requirement for voluntary consent [87] [88]. The Declaration of Helsinki further refined these concepts by distinguishing between therapeutic and non-therapeutic research and introducing the concept of oversight by independent committees [89]. The Belmont Report synthesized and built upon these foundations in response to ethical failures in the United States, most notably the Tuskegee Syphilis Study, creating a principled framework that has demonstrated remarkable resilience and relevance for over four decades [7] [13].
Historical Context and Development
Nuremberg Code (1947)
The Nuremberg Code originated from the post-World War II trials of Nazi physicians who conducted brutal experiments on concentration camp prisoners without their consent [87] [88]. Created by the Nuremberg Military Tribunal during the U.S. v Brandt case, this 10-point code was the first international document to delineate permissible medical experimentation on humans [85] [88]. The Code's primary contribution was its emphatic assertion that "the voluntary consent of the human subject is absolutely essential" [85]. This principle established the inviolable nature of informed consent in research ethics, though the Code itself never received formal adoption as law by any nation [87]. The Nuremberg Code emerged specifically as a response to egregious war crimes and was initially viewed by many in the medical community as a standard for "barbarians" rather than ordinary researchers, limiting its immediate impact on day-to-day research practice [87].
Declaration of Helsinki (1964)
The World Medical Association (WMA) developed the Declaration of Helsinki as a more practical set of ethical guidelines for physicians engaged in medical research [86] [89]. First adopted in 1964 and subsequently revised multiple times (most recently in 2024), the Declaration built upon the Nuremberg Code while introducing several critical advancements [86] [90]. It formally distinguished between clinical research combined with professional care and non-therapeutic research, establishing different ethical standards for each category [89]. The Declaration also introduced the requirement for independent committee review of research protocols, a precursor to modern institutional review boards (IRBs) [86] [89]. Unlike the Nuremberg Code, which was created by jurists, the Declaration of Helsinki was developed by physicians for physicians, giving it greater practical acceptance within the medical community [89]. Its most recent 2024 revision emphasizes including vulnerable groups in research while ensuring appropriate protections, reflecting evolving ethical considerations [90].
Belmont Report (1979)
The Belmont Report emerged from the work of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, created by the U.S. Congress in part as a response to the Tuskegee Syphilis Study [7] [13]. Published in 1979, the Report provided the ethical foundation for what would become the U.S. Federal Policy for the Protection of Human Subjects, known as the "Common Rule" [7]. The Belmont Report's significant contribution was its articulation of three fundamental ethical principles: Respect for Persons, Beneficence, and Justice [7] [13]. Rather than presenting a simple list of rules, the Report organized ethical requirements into a principled framework that could be applied to novel research contexts, making it particularly valuable for addressing emerging ethical challenges in research [7]. The Report's principles have been incorporated into fundamental training for clinical researchers worldwide and continue to influence international guidelines, including the recently updated International Council for Harmonisation's Guideline for Good Clinical Practice E6(R3) [7].
Table: Historical Development of Key Research Ethics Documents
| Document | Year Created | Primary Catalyst | Key Contributions |
|---|---|---|---|
| Nuremberg Code | 1947 | Nazi medical experiments | Established absolute requirement for voluntary consent; First code for human experimentation |
| Declaration of Helsinki | 1964 (revised 2024) | Need for physician-specific guidelines | Distinguished therapeutic/non-therapeutic research; Required independent review; Emphasized patient welfare |
| Belmont Report | 1979 | Tuskegee Syphilis Study & other U.S. research abuses | Three-principle framework; Basis for U.S. regulations; Application to behavioral & biomedical research |
Comparative Ethical Principles Analysis
Foundational Ethical Frameworks
The Nuremberg Code, Declaration of Helsinki, and Belmont Report approach research ethics from distinct conceptual foundations. The Nuremberg Code presents ten direct principles focused specifically on the conditions under which human experimentation is permissible, with its primary emphasis on voluntary consent and the researcher's responsibility to protect subjects [85] [88]. The Declaration of Helsinki offers a more comprehensive set of guidelines specifically for physicians, emphasizing the distinction between research and care, and introducing the concept of oversight by independent committees [86] [89]. The Belmont Report differs fundamentally by presenting a three-principle framework (Respect for Persons, Beneficence, and Justice) that is intended to be applied analytically to research situations rather than providing specific prescriptions [7] [13].
This conceptual difference makes the Belmont Report particularly adaptable to novel research contexts that its drafters could not have anticipated. As noted by James Riddle, an expert in research ethics, "the Belmont Report has stood the test of time since its earliest days of formation... right up to the present" because its principled approach allows for application to evolving research methodologies [7]. The Nuremberg Code and Declaration of Helsinki, while foundational, operate as more specific sets of rules rather than as analytical frameworks.
Informed Consent Requirements
All three documents address informed consent, but with varying emphasis and specificity. The Nuremberg Code establishes the most absolute standard, stating that "the voluntary consent of the human subject is absolutely essential" and detailing the elements required for consent to be considered valid, including legal capacity, free power of choice, and sufficient knowledge [85] [88]. The Declaration of Helsinki expands on these concepts, requiring that potential subjects be informed of "aims, methods, anticipated benefits and potential risks and burdens, qualifications of the researcher, sources of funding, any potential conflicts of interest," and other relevant aspects [86]. It also specifically addresses consent in vulnerable populations and situations where potential participants may be in dependent relationships with researchers [86].
The Belmont Report approaches consent through the principle of Respect for Persons, which encompasses two ethical convictions: that individuals should be treated as autonomous agents, and that persons with diminished autonomy are entitled to protection [13]. This principled approach to consent allows for greater flexibility in application while maintaining the fundamental requirement that subjects must understand the research and volunteer voluntarily. The Report emphasizes that information must be comprehended, consent must be voluntary, and the process should be tailored to the subject's capacities [13].
Risk-Benefit Assessment
The assessment of risks and benefits is treated differently across the three documents. The Nuremberg Code states that experiments should avoid all unnecessary physical and mental suffering, that no experiment should be conducted if there is a priori reason to believe death or disabling injury will occur, and that the degree of risk should never exceed the humanitarian importance of the problem [85] [88]. The Declaration of Helsinki requires careful assessment of predictable risks and burdens compared to foreseeable benefits, with continuous monitoring and documentation by the researcher [86]. It specifically states that medical research may only be conducted if "the importance of the objective outweighs the risks and burdens to the research participants" [86].
The Belmont Report addresses this area through the principle of Beneficence, which extends beyond non-maleficence to an obligation to secure the well-being of research participants [13]. This principle is operationalized through a systematic assessment of risks and benefits, requiring that researchers maximize possible benefits and minimize possible risks [13]. The Belmont approach recognizes that while risks cannot be entirely eliminated, they must be justified by the potential benefits to subjects or society, and must be constantly monitored throughout the research process [13].
Table: Comparison of Core Ethical Requirements
| Ethical Element | Nuremberg Code | Declaration of Helsinki | Belmont Report |
|---|---|---|---|
| Consent Foundation | Absolute voluntary consent | Free and informed consent | Respect for autonomy |
| Risk-Benefit Standard | Risk justified by humanitarian importance | Risks justified by potential benefits | Systematic assessment of risks and benefits |
| Vulnerable Populations | Not specifically addressed | Specific protections and inclusion considerations | Additional protections for those with diminished autonomy |
| Oversight Mechanism | Researcher responsibility | Independent ethics committee review | Institutional review boards (IRBs) |
| Scope | Medical experimentation | Medical research involving human subjects | All research involving human subjects |
Application in Modern Research Settings
Implementation in Regulatory Frameworks
The principles articulated in these documents have been translated into concrete regulatory requirements that govern modern research practice. The Belmont Report directly influenced the creation of the U.S. Federal Policy for the Protection of Human Subjects (the "Common Rule") found in 45 CFR part 46, which outlines the duties of institutional review boards (IRBs) and the requirements for informed consent [7]. These regulations are enforced through IRBs that review research protocols to ensure ethical standards are met [87] [13]. The Declaration of Helsinki has influenced international guidelines, including the International Ethical Guidelines for Biomedical Research Involving Human Subjects proposed by the World Health Organization and the Council for International Organizations of Medical Sciences (CIOMS) [91] [92]. While the Nuremberg Code has not been officially adopted as law by any nation, its principles have been incorporated into international human rights instruments, such as the International Covenant on Civil and Political Rights, and have been referenced in U.S. court decisions [87].
Influence on Good Clinical Practice (GCP)
The principles from all three documents have been synthesized into Good Clinical Practice (GCP) guidelines that govern the conduct of clinical trials worldwide [88]. As noted in the search results, "the goal of GCP—as was the goal of the Nuremberg Code—was and will always be the protection of human subjects that are part of all ethical clinical trials" [88]. The recently updated International Council for Harmonisation's Guideline for Good Clinical Practice E6(R3) maintains strong ties to the ethical foundations laid out in the Belmont Report and other foundational documents [7]. For drug development professionals, this means that understanding the ethical principles in these historical documents is essential for implementing GCP standards in daily research practice.
Contemporary Ethical Challenges
The principled framework of the Belmont Report provides particular value in addressing emerging ethical challenges in modern research, including research with biologial samples and data, international research in resource-poor settings, and studies involving vulnerable populations [86] [13]. The Declaration of Helsinki's 2024 revision specifically addresses some of these issues by encouraging "the inclusion of vulnerable groups and individuals in clinical research, protecting them through research' instead of from research" [90]. The Belmont Report's principle of Justice, which requires fair distribution of the burdens and benefits of research, provides ethical guidance for ensuring that research populations are not exploited and that the benefits of research reach those who bear its burdens [13].
The Scientist's Toolkit: Essential Components for Ethical Research Implementation
Table: Key Research Ethics Resources and Their Applications
| Resource | Function | Application in Research Practice |
|---|---|---|
| Informed Consent Documents | Ensure participants' autonomous decision-making | Document comprehension of study purpose, risks, benefits, and alternatives |
| Protocol Templates | Provide ethical framework for study design | Incorporate risk minimization, fair subject selection, and data confidentiality |
| IRB/EC Submission Systems | Facilitate independent ethical review | Ensure oversight by qualified, diverse committees with sufficient resources |
| Safety Monitoring Plans | Implement beneficence principle | Systematically assess, monitor, and document risks throughout study |
| Vulnerability Assessment Tools | Apply justice principle | Identify needs of vulnerable populations for additional protections |
| Ethics Training Modules | Build researcher competency | Provide education on historical foundations and contemporary applications |
Visualizing the Ethical Framework Relationship
Diagram: The Historical Evolution and Application of Research Ethics Frameworks. This visualization shows how ethical concepts developed sequentially, with each document building upon its predecessors, and how they collectively inform modern research practices.
The comparative analysis of the Belmont Report against the Nuremberg Code and Declaration of Helsinki reveals a clear evolutionary pathway in research ethics, from specific rules developed in response to historical abuses toward a principled framework capable of addressing novel ethical challenges. For today's researchers, scientists, and drug development professionals, the Belmont Report's three principles of Respect for Persons, Beneficence, and Justice provide the most comprehensive and adaptable foundation for designing, conducting, and evaluating research involving human subjects [7] [13]. While the Nuremberg Code established the non-negotiable requirement of voluntary consent and the Declaration of Helsinki introduced critical distinctions between research and care, the Belmont Report's framework has demonstrated remarkable resilience and relevance for over four decades [7].
The ongoing development of ethical standards—exemplified by the recent 2024 revision of the Declaration of Helsinki—continues to be informed by these foundational documents [90]. As research methodologies evolve with technological advancements, the principled approach of the Belmont Report ensures that ethical considerations remain central to the research enterprise. For the scientific community, understanding this ethical lineage is not merely about regulatory compliance but about maintaining public trust and upholding the fundamental dignity and rights of those who volunteer to participate in research. The Belmont Report's principles continue to serve as the essential ethical compass guiding researchers through the complex moral landscape of human subjects research.
This whitepaper examines the enduring relevance of principle-based frameworks in bioethics, with a specific focus on the Belmont Report's foundational principles and their application in modern research contexts, including drug development and artificial intelligence. Despite emerging critiques regarding vagueness in implementation and integration challenges, principles-based approaches continue to provide essential ethical guidance that adapts to technological innovation. We analyze the hierarchical application of ethical principles from theoretical foundations to practical governance, document empirical research on their implementation, and propose structured methodologies for addressing integration challenges in contemporary research environments. The analysis demonstrates that when supplemented with appropriate safeguards and implementation frameworks, principles-based approaches maintain critical relevance for navigating complex ethical landscapes in scientific research.
Principles-based approaches represent a fundamental methodology in bioethics that relies on broad ethical standards to guide decision-making, contrasting with rules-based regulation that depends on specific prescriptive requirements [93]. This approach has deep roots in medical ethics history, with principles of beneficence and nonmaleficence traceable to the Hippocratic tradition, while autonomy and justice gained prominence later in the ethical discourse [49]. The most influential articulation of this framework in research ethics emerged in the Belmont Report of 1979, which established three core principles—Respect for Persons, Beneficence, and Justice—to govern research involving human subjects [3].
The transition toward principles-based approaches responded to historical ethical failures in research, including deplorable abuses of human subjects in the World War II era and medical interventions conducted without informed consent [3]. These incidents revealed the limitations of existing ethical frameworks and prompted the development of more robust guidance. The Belmont Report's principles subsequently formed the ethical foundation for regulations and ethical review processes, including Institutional Review Boards (IRBs) [3].
In contemporary contexts, principles-based approaches face both new challenges and opportunities for application. Technological advances in health data quality, availability, and linkage potential create novel ethical dilemmas that strain traditional governance mechanisms [93]. Similarly, the integration of artificial intelligence (AI) in medicine introduces ethical challenges regarding algorithmic bias, discrimination against patients, and potential misdiagnoses [94]. This whitepaper examines how principles-based frameworks adapt to these evolving research landscapes while maintaining their foundational ethical commitments.
The Belmont Report's Foundational Principles
The Belmont Report, formally titled "Ethical Principles and Guidelines for the Protection of Human Subjects of Research," established three fundamental principles that continue to govern human subjects research in the United States and internationally [3]. These principles emerged from the work of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, created by the Department of Health, Education, and Welfare in response to the National Research Act of 1974 [3].
The Three Core Principles
Respect for Persons: This principle acknowledges the intrinsic worth and autonomy of individuals and requires that research subjects enter into the research process voluntarily and with adequate information. It supports the practice of informed consent and protects individuals with diminished autonomy [3]. The philosophical underpinning of this principle draws from the work of Immanuel Kant and John Stuart Mill, who asserted that all persons have unconditional worth and should have the power to make rational decisions and moral choices [49].
Beneficence: This principle extends beyond the Hippocratic tradition of "do no harm" to encompass an affirmative obligation to maximize benefits and minimize potential harms to research subjects. It requires researchers to systematically assess risks and benefits and ensure that the potential benefits justify the risks [3]. In clinical ethics, this principle is often paired with nonmaleficence (avoiding harm), creating a dual obligation to benefit patients and avoid causing harm [49].
Justice: This principle addresses the fair distribution of research burdens and benefits across society. It requires that researchers not systematically select vulnerable populations for risky research while reserving the benefits for more affluent populations [3]. The principle demands special attention to protecting socially vulnerable groups, including children, prisoners, and adults without decision-making capacity [3].
Applications in Research Practice
The Belmont Report translated these abstract principles into concrete applications for research practice:
Informed Consent: Flowing from Respect for Persons, informed consent requires that subjects be given adequate information, comprehend the disclosure, and participate voluntarily without coercion [49]. The Report acknowledges that the specific requirements may need adaptation across different cultural contexts while maintaining core protections [49].
Assessment of Risks and Benefits: Derived from the principle of Beneficence, this application requires a systematic analysis of the potential harms and benefits of research, with the goal of maximizing benefits while minimizing risks [3].
Selection of Subjects: Based on the principle of Justice, this application addresses the fair procedures and outcomes in selecting research subjects, with particular attention to avoiding the exploitation of vulnerable populations [3].
Table 1: Belmont Report Principles and Applications
| Ethical Principle | Core Meaning | Research Application | Vulnerabilities Protected |
|---|---|---|---|
| Respect for Persons | Recognition of personal autonomy and protection for those with diminished autonomy | Informed consent process | Individuals with impaired decision-making capacity |
| Beneficence | Obligation to maximize benefits and minimize harms | Systematic assessment of risks and benefits | All research subjects exposed to potential harms |
| Justice | Fair distribution of research burdens and benefits | Equitable selection of subjects | Socially vulnerable groups |
Principles-Based Approaches in Contemporary Research Ethics
Hierarchical Framework for Empirical Research
Principles-based approaches operate at multiple levels in contemporary research ethics. A hierarchical framework categorizes empirical research in bioethics into four distinct levels [95]:
Lay of the Land Studies: These investigations define current practices, opinions, beliefs, or other aspects of the status quo. They answer questions such as "What do physicians think about X?" or "How do nurses perceive Y?" [95]. This research sets the stage for further investigation and helps direct efforts in improving care.
Ideal Versus Reality Studies: This research begins with a premise regarding ethical norms and assesses the extent to which actual clinical practice reflects this ideal. Examples include studies demonstrating that racial and ethnic minority patients receive less aggressive and lower quality care despite the ethical norm of equality [95].
Improving Care Studies: This level moves beyond assessment to address how we can bring clinical practice closer in line with ethical ideals. It involves developing and testing interventions to improve ethical practice [95].
Changing Ethical Norms: The highest level of work brings together data from multiple empirical studies on a single topic and uses these data to inform and potentially change ethical ideals. This represents the most direct way empirical research can influence normative frameworks [95].
Principles Versus Rules-Based Approaches
A significant development in principles-based approaches is the distinction between principle-based regulation (PBR) and rules-based regulation (RBR). While RBR relies on compliance with specific, prescriptive rules, PBR depends on broadly stated objectives, standards, and values [93]. This distinction has important practical implications:
Table 2: Principles-Based vs. Rules-Based Regulation
| Characteristic | Principles-Based Regulation (PBR) | Rules-Based Regulation (RBR) |
|---|---|---|
| Foundation | Broadly-stated objectives and values | Specific prescriptive rules |
| Flexibility | High - adapts to unanticipated situations | Low - rigid in novel circumstances |
| Decision-Making | Encourages reflection and justification | Promotes tick-box compliance mentality |
| Conflict Resolution | Appeals to underlying values and objectives | Requires additional rules for clarification |
| Cultural Impact | Fosters culture of reflection | Creates culture of mere compliance |
Principles-based approaches are particularly suited to the health research sector, which must balance two central public interests: (1) promoting scientifically sound, ethically robust research, and (2) securing adequate protection for citizens' privacy when their data are used for research [93]. Individualistic governance devices such as consent and anonymization prove inadequate alone for addressing this balance, necessitating a principles-based approach that can accommodate nuanced judgments [93].
Critiques and Implementation Challenges
Vagueness and Methodological Challenges
Despite their widespread adoption, principles-based approaches face significant critiques regarding their implementation. Empirical research with bioethics scholars reveals an air of uncertainty and overall vagueness that surrounds integration methods [96]. This indeterminacy presents both advantages and disadvantages—allowing flexibility while potentially obscuring a lack of understanding of theoretical-methodological underpinnings [96].
The integration of empirical data with normative analysis remains particularly challenging. A systematic review identified thirty-two distinct methodologies for integration, categorized as dialogical, consultative, or combined approaches [96]. This methodological diversity creates uncertainty about aims, content, and domain of application. Furthermore, the steps guiding integration processes are often unspecific and frustratingly vague in practical contexts [96]. For instance, in reflective equilibrium—a common integration method—pressing issues exist regarding how much weight should be given to empirical data versus ethical theory [96].
Structural and Operational Challenges
Empirical research with research staff working in global development contexts identifies structural challenges in implementing ethical principles [40]. Research environments in the 'Global South' often pose particular challenges that, if unaddressed, can result in insecurity, sexual harassment, emotional distress, exploitative employment conditions, and discrimination [40]. These findings suggest that structural asymmetries serve as a key driver of ethical challenges, requiring solutions at multiple levels rather than merely focusing on individual compliance [40].
The diagram below illustrates the structural challenges in implementing principles-based approaches and their impacts on research integrity:
Structural Challenges in Principles Implementation
Cultural and Contextual Limitations
Principles-based approaches face challenges in cross-cultural application. The emphasis on individual autonomy in Western bioethics may conflict with family-centered or community-based decision-making traditions in other cultures [49]. Even within Western contexts, minority populations may hold views different from the majority white population regarding needs for full disclosure and decisions about life support [49].
Detractors of the principle of autonomy question its individualistic focus and propose a broader concept of relational autonomy shaped by social relationships and complex determinants such as gender, ethnicity, and culture [49]. This critique highlights the need for principles-based approaches to accommodate diverse cultural perspectives while maintaining core ethical protections.
Application in Drug Development and Medical Innovation
Ethical Frameworks for Drug Development
The principles-based approach has significant applications in drug development, where high failure rates (approximately 90% of clinical drug development fails) raise ethical concerns about resource allocation and risk-benefit ratios [97]. Current drug optimization processes overly emphasize potency and specificity while overlooking tissue exposure and selectivity, potentially misleading candidate selection and impacting the balance of clinical dose, efficacy, and toxicity [97].
A proposed Structure–Tissue Exposure/Selectivity–Activity Relationship (STAR) framework classifies drug candidates based on potency/selectivity, tissue exposure/selectivity, and required dose for balancing clinical efficacy/toxicity [97]. This approach exemplifies how principles-based frameworks can guide ethical decision-making in complex technical domains:
Table 3: STAR Framework for Ethical Drug Development
| Drug Class | Specificity/Potency | Tissue Exposure/Selectivity | Clinical Dose | Ethical Risk-Benefit Profile |
|---|---|---|---|---|
| Class I | High | High | Low | Superior clinical efficacy/safety with high success rate |
| Class II | High | Low | High | High toxicity requiring cautious evaluation |
| Class III | Adequate | High | Low | Manageable toxicity but often overlooked |
| Class IV | Low | Low | Variable | Inadequate efficacy/safety - should be terminated early |
Artificial Intelligence in Medicine
Principles-based approaches are increasingly applied to ethical challenges in artificial intelligence (AI) in medicine. A principle-based approach to teaching AI ethics in medical education builds on the four principles of medical ethics—autonomy, beneficence, nonmaleficence, and justice—and extends them by integrating three public health ethics principles: efficiency, common good orientation, and proportionality [94].
This approach addresses unique ethical challenges posed by AI, including:
Algorithmic Bias: AI systems trained on unrepresentative data may perpetuate or amplify existing healthcare disparities, violating principles of justice [94].
Transparency and Accountability: The "black box" nature of some AI algorithms creates challenges for informed consent and physician responsibility [94].
Clinical Validity: The low accuracy and validity of some AI systems may undermine utility and trust in clinical applications [94].
The diagram below illustrates the extended principles framework for AI ethics in medicine:
Extended Principles Framework for AI Ethics
Methodological Approaches for Empirical Bioethics Research
Researcher Perspectives on Empirical-Normative Integration
Empirical research with bioethics scholars reveals diverse methodological approaches to integrating empirical data with normative analysis. Researchers report using various methodologies, including [96]:
Back-and-Forth Methods: Primarily reflective equilibrium, where researchers move iteratively between empirical data and normative principles to achieve moral coherence.
Dialogical Methods: Approaches that emphasize collaboration and stakeholder dialogue as a means of integration.
Inherent Integration Approaches: Methods where the normative and empirical dimensions are intertwined from the project's inception.
Despite this methodological diversity, researchers report uncertainty and vagueness in implementation. This suggests a need for more structured approaches to integration while maintaining methodological flexibility.
Acceptable Objectives for Empirical Bioethics Research
Research with empirical bioethics scholars has identified a hierarchy of acceptability for various research objectives. From eight proposed objectives, understanding the context of a bioethical issue and identifying ethical issues in practice received unanimous agreement [98]. Other objectives, such as evaluating how ethical recommendations work in practice and recommending changes in ethical norms, received varying degrees of support [98].
The most contested objectives were striving to draw normative recommendations and developing and justifying moral principles [98]. This hierarchy suggests that empirical bioethics researchers are most comfortable with descriptive and diagnostic applications of empirical research and more cautious about prescriptive applications that directly challenge or modify existing normative frameworks.
The principles-based approach, despite valid critiques regarding implementation challenges and methodological vagueness, maintains enduring relevance in modern bioethics. The foundational principles articulated in the Belmont Report continue to provide essential guidance for research ethics, particularly as technological innovations create novel ethical dilemmas [3] [94]. The flexibility of principles-based approaches represents both their greatest strength and most significant challenge—allowing adaptation to diverse contexts while creating implementation uncertainties [93] [96].
For researchers, scientists, and drug development professionals, principles-based frameworks offer essential guidance for navigating complex ethical landscapes. The integration of empirical research with normative analysis, while methodologically challenging, strengthens the relevance and applicability of ethical guidance to real-world contexts [95] [96]. Future developments should focus on creating more structured implementation frameworks while preserving the flexibility that makes principles-based approaches valuable across diverse research contexts.
As technological advances in medicine continue to accelerate, principles-based approaches will remain essential for addressing novel ethical challenges while maintaining foundational commitments to human dignity, wellbeing, and justice. The continued evolution of these frameworks will require ongoing dialogue between empirical researchers, normative scholars, and scientific professionals to ensure their relevance and effectiveness in guiding ethical research practices.
The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research stands as a foundational document in the landscape of research ethics. Published in 1979 by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, this report was forged from a necessary response to egregious ethical violations in research, most notably the Tuskegee Syphilis Study [99] [2]. Congress passed the National Research Act of 1974, leading to the Commission's formation and its ultimate charge: to identify the basic ethical principles that should underpin the conduct of research involving human subjects [99] [14]. Despite being nearly five decades old, the Belmont Report's framework remains remarkably timely, continuing to shape federal regulations, institutional review processes, and even contemporary discussions around emerging fields like artificial intelligence [7] [100].
This document provides an in-depth technical guide for researchers, scientists, and drug development professionals, focusing on how the Belmont Report's foundational principles provide the ethical backbone for modern research conduct. The Report itself was intended not as a rigid checklist, but as an analytical framework to guide researchers and review boards in their ethical deliberations [2]. Its enduring legacy is codified in the Federal Policy for the Protection of Human Subjects, commonly known as the "Common Rule" (45 CFR 46), which governs nearly all U.S. federally-supported human subjects research [7] [1]. By understanding the Belmont principles and their applications, research professionals can ensure their work not only complies with federal regulations but also aligns with the highest standards of ethical integrity.
Historical and Regulatory Context of the Belmont Report
Pre-Belmont Ethical Milestones
The creation of the Belmont Report did not occur in an ethical vacuum. It was preceded by critical international documents that first attempted to establish boundaries for human experimentation. The Nuremberg Code, formulated in 1947 in the aftermath of the Nazi doctors' trials, was a pivotal response to atrocities committed in the name of research [3] [12]. Its first principle, the absolute necessity of voluntary consent, established autonomy as a paramount concern, though the Code's context was limited by its focus on prisoners in concentration camps [3]. Subsequently, the Declaration of Helsinki, first adopted by the World Medical Association in 1964, distinguished between clinical research combined with professional care and non-therapeutic clinical research [3]. It emphasized the principle of Beneficence and introduced the concept of ethical review by an independent committee, a precursor to the modern Institutional Review Board (IRB) [3].
However, these guidelines proved insufficient to prevent ethical abuses within the United States. The Tuskegee Syphilis Study, conducted by the U.S. Public Health Service, became the catalyst for national reform [99]. In this study, which lasted from 1932 to 1972, Black men with syphilis were deceived and denied effective treatment so that researchers could observe the natural progression of the disease [99]. The public revelation of this study in 1972 shocked the nation and revealed a stark flaw in existing human subjects protection policies, ultimately prompting Congress to pass the National Research Act of 1974 [99].
Incorporation into Federal Regulation
The National Commission's work culminated in the 1979 Belmont Report, which provided the ethical foundation for a comprehensive regulatory structure. The Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) subsequently revised and expanded their regulations, embedding the Report's principles into the core of U.S. research governance [12]. These regulations are now encapsulated in the Common Rule (45 CFR 46) and various FDA regulations (e.g., 21 CFR 50, 56) [12]. It is crucial to note that while these principles are legally binding for government-funded research, their adoption in the private sector remains a matter of choice, a point of significant relevance in today's era of industry-led AI and drug development [100].
Table: Historical Progression of Research Ethics Guidelines
| Document/Event | Year | Key Ethical Contribution | Primary Catalyst |
|---|---|---|---|
| Nuremberg Code | 1947 | Established the absolute requirement for voluntary informed consent | Nazi human experimentation during WWII [12] [99] |
| Declaration of Helsinki | 1964 | Distinguished therapeutic vs. non-therapeutic research; emphasized beneficence and independent ethical review [3] | Growing international consensus on medical ethics [3] |
| Tuskegee Syphilis Study Revelation | 1972 | Exposed ethical failures in long-term U.S. research; demonstrated harm from deceptive practices and lack of informed consent [99] | Associated Press investigation and public outrage [99] |
| National Research Act | 1974 | Mandated the creation of the National Commission to identify ethical principles for human subjects research [2] [99] | Public and Congressional response to Tuskegee and other ethical breaches [99] |
| The Belmont Report | 1979 | Articulated the three core principles of Respect for Persons, Beneficence, and Justice and their applications [1] | Work of the National Commission as required by the National Research Act [2] |
| The Common Rule (45 CFR 46) | 1981 (Revised 2017) | Codified the Belmont principles into federal regulations for the protection of human subjects [7] [1] | Need for a uniform federal policy based on the ethical framework of the Belmont Report [3] [7] |
Foundational Ethical Principles and Their Applications
The Belmont Report establishes three fundamental ethical principles that are now universally recognized as the cornerstone for the ethical conduct of research involving human subjects. These are: Respect for Persons, Beneficence, and Justice [1] [2]. The Report further translates these abstract principles into concrete applications in research practice, primarily through Informed Consent, Assessment of Risks and Benefits, and Selection of Subjects [3] [1].
Principle 1: Respect for Persons
The principle of Respect for Persons incorporates two distinct ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection [1]. This principle acknowledges the right of individuals to make their own decisions and to have those choices respected.
Application in Informed Consent: To satisfy this principle, researchers must ensure that subjects enter into the research voluntarily and with adequate information [1]. This involves providing a clear explanation of the research procedures, their purposes, potential risks and anticipated benefits, alternative procedures, and a statement offering the subject the opportunity to ask questions and to withdraw from the research at any time without penalty [1]. The consent process must be free from coercion or undue influence, and the information must be presented in a language and manner that is comprehensible to the subject [1] [14].
Protection of Vulnerable Populations: The second moral requirement under this principle obliges researchers to protect individuals with diminished autonomy. This includes, but is not limited to, children, prisoners, individuals with cognitive impairments, and others who may be vulnerable to coercion or incapable of fully autonomous decision-making [1] [14]. The extent of protection should be commensurate with the risk of harm and the likelihood of benefit. For example, research with children requires parental permission and often the assent of the child, honoring their developing autonomy to the extent possible [14].
Principle 2: Beneficence
The principle of Beneficence extends beyond merely refraining from harm; it imposes an obligation to maximize possible benefits and minimize possible harms [1] [2]. This principle is often summarized by two complementary rules: "(1) do not harm and (2) maximize possible benefits and minimize possible harms" [1].
- Application in Systematic Risk/Benefit Assessment: Researchers and IRBs must conduct a thorough and systematic analysis of the proposed research to identify all potential risks and benefits [1]. This assessment must be factual, rigorous, and non-arbitrary, considering not only physical harm but also psychological, social, and economic risks [1]. The goal is to determine whether the potential benefits to the individual subject or to society justify the risks posed to the subjects. As the University of Washington's guidance notes, if there are any risks from participation, there must be benefits, either to the subject or to humanity/society in general [14].
Principle 3: Justice
The principle of Justice requires the fair distribution of the burdens and benefits of research [2]. It addresses the ethical concern that certain classes or groups of individuals should not be systematically selected for research simply because of their easy availability, compromised position, or societal marginalization [1].
- Application in Equitable Subject Selection: This principle demands that researchers scrutinize their inclusion and exclusion criteria to avoid systemic biases [1]. Historically, burdens of research have disproportionately fallen upon poor, racial minorities, and institutionalized populations, while the benefits of advanced medical care have primarily flowed to more privileged groups [2]. The application of justice requires that no single group should be unfairly burdened with the risks of research, nor should any group be unfairly excluded from the potential benefits of participation [2] [14]. For instance, the inclusion of women and minorities in clinical trials is now a matter of regulatory expectation to ensure that the resulting knowledge and therapies are effective for all segments of the population.
Table: The Belmont Report's Ethical Principles and Corresponding Applications
| Ethical Principle | Core Meaning | Research Application | Key Questions for Researchers |
|---|---|---|---|
| Respect for Persons | Individuals are autonomous agents; those with diminished autonomy deserve special protection [1]. | Informed Consent [3] [1] | Is consent voluntary and informed? Are vulnerable populations given adequate protection? Is confidentiality maintained? [1] |
| Beneficence | An obligation to maximize benefits and minimize harms and wrongs [1] [2]. | Assessment of Risks and Benefits [3] [1] | Are the risks justified by the benefits? Are risks minimized to the extent possible? Is the research design sound? [1] |
| Justice | Fairness in the distribution of research burdens and benefits [1] [2]. | Selection of Subjects [3] [1] | Are subjects selected for reasons directly related to the problem, not for convenience or manipulability? Is there a fair selection process? [1] |
Methodologies and Experimental Protocols for Ethical Review
The Belmont Report not only provides principles but also implies a methodological framework for ethical analysis. Institutional Review Boards (IRBs) use this framework to systematically evaluate research protocols. The following workflow and toolkit outline this process.
Ethical Review Workflow
The following diagram visualizes the systematic, non-arbitrary method that IRB members use to determine if the risks of a research study are justified by the benefits, as informed by the Belmont principles [1].
Ethical Review Workflow for Research Protocols
The Researcher's Toolkit: Essential Components for Ethical Research
For researchers designing a study, adherence to the Belmont principles requires specific "tools" or components to be integrated into the experimental protocol. The following table details these essential elements.
Table: Research Reagent Solutions for Ethical Study Design
| Toolkit Component | Function in the Research Protocol | Relevant Belmont Principle |
|---|---|---|
| Informed Consent Document (ICD) | A comprehensive document that provides all relevant information about the study (purpose, procedures, risks, benefits, alternatives) in an understandable language to enable a potential subject's voluntary decision to participate [1]. | Respect for Persons [1] |
| Institutional Review Board (IRB) Application | The formal submission to an independent ethical review committee that details the study's design, subject population, risks, benefits, and consent process, ensuring compliance with ethical principles and federal regulations [12] [99]. | Respect for Persons, Beneficence, Justice [1] |
| Data Safety and Monitoring Plan (DSMP) | A proactive plan to ensure subject safety and data integrity during the trial. It outlines procedures for monitoring, auditing, and reporting adverse events, thereby minimizing harm [1]. | Beneficence [1] |
| Inclusion/Exclusion Criteria Justification | A scientifically sound rationale for the selection of the study population, designed to ensure fair subject selection and avoid the systematic exploitation of vulnerable groups [1] [2]. | Justice [1] |
| Vulnerable Population Safeguards | Additional protective measures (e.g., assent forms for children, surrogate consent procedures for cognitively impaired individuals, additional oversight for prisoners) tailored to specific vulnerable groups [1] [14]. | Respect for Persons [1] |
| Risk/Benefit Analysis Matrix | A systematic, factual analysis that itemizes and evaluates all foreseeable risks (physical, psychological, social, economic) and balances them against anticipated benefits to the subject and/or society [1]. | Beneficence [1] |
Contemporary Applications and Future Directions
The ethical framework of the Belmont Report has proven to be remarkably adaptable, extending its relevance beyond traditional biomedical research into new and emerging fields. Its principles provide a stable foundation for navigating the complex ethical terrain of modern technology and global research.
The Belmont Report in the Age of Artificial Intelligence
Recent scholarship from institutions like the National Institute of Standards and Technology (NIST) suggests that the Belmont Report provides a crucial historical precedent for guiding ethical research in Artificial Intelligence (AI) [100]. The core principles translate effectively to the challenges posed by AI development. For example, the principle of Respect for Persons requires informed consent for the use of personal data; this is directly relevant when AI systems are trained on data scraped from the web without users' knowledge or permission [100]. Beneficence, the obligation to minimize harm, applies directly to the need to identify and mitigate algorithmic biases that can lead to discriminatory outcomes in hiring, lending, and law enforcement [100]. Finally, Justice is a critical lens through which to view the problem of biased training data. If a facial recognition system is trained primarily on one demographic, it performs poorly on others, leading to a disproportionate distribution of harm—a clear violation of the justice principle that can be avoided through equitable data selection practices [100].
Balancing Principles in Complex Research Scenarios
The Belmont principles are not always easy to apply and can sometimes conflict, requiring careful deliberation. Research involving children provides a clear example of such a conflict. While the principle of Respect for Persons requires honoring a child's dissent, the principles of Beneficence (securing a potential medical benefit) and Justice (ensuring children benefit from research) may, in specific and highly regulated circumstances, justify a parent's decision to override that dissent for a child's potential direct benefit [14]. Resolving such conflicts does not involve discarding one principle for another, but rather a careful, contextual balancing act overseen by the IRB, demonstrating the framework's role as a dynamic guide for ethical decision-making rather than a rigid set of rules [2] [14].
The Belmont Report endures because its three core principles—Respect for Persons, Beneficence, and Justice—articulate a profound and universal truth about the ethical conduct of research. They provide a robust and flexible "compass" rather than a simple checklist, allowing the framework to remain relevant across decades of scientific and societal change [2]. As this guide has detailed, these principles are not abstract ideas; they are operationalized into concrete applications—Informed Consent, Risk/Benefit Assessment, and Equitable Subject Selection—that form the bedrock of federal regulations like the Common Rule and the daily workings of IRBs [3] [7] [1].
For researchers, scientists, and drug development professionals, a deep understanding of the Belmont Report is not merely a regulatory requirement but a fundamental component of professional integrity. By internalizing and applying this ethical framework, the research community can uphold the highest standards of practice, maintain public trust, and ensure that the pursuit of scientific progress is always aligned with the protection of human dignity and rights. Its successful application to cutting-edge fields like AI further validates the Report's power as a lasting standard for ethical research conduct [100].
Conclusion
The Belmont Report remains the foundational compass for ethical research, providing a robust and flexible framework of Respect for Persons, Beneficence, and Justice that is more critical than ever in today's rapidly advancing biomedical landscape. For researchers and drug development professionals, these principles are not historical artifacts but active tools that guide study design, inform daily decisions, and ensure that scientific progress is matched by a steadfast commitment to human dignity and rights. The key takeaway is that ethical rigor and scientific excellence are inseparable. As we move into the future of gene editing, AI in healthcare, and personalized medicine, the Belmont Report's principles will continue to be essential for navigating new ethical frontiers, fostering public trust, and directing innovation toward equitable and morally sound outcomes.
References
- 1. Belmont Report - Human Research Protection Program [https://irb.wisc.edu/regulatory-information/belmont-report/]
- 2. The Belmont Report - Human Subjects Office [https://hso.research.uiowa.edu/about-human-subjects-office/regulations-and-policies/belmont-report]
- 3. The creation of the Belmont Report and its effect on ethical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9700634/]
- 4. FDA Roundup: January 7, 2025 [https://www.fda.gov/news-events/press-announcements/fda-roundup-january-7-2025]
- 5. NIH Follows in FDA's Footsteps and Adopts “Bulk Sensitive ... [https://www.mofo.com/resources/insights/251028-nih-adopts-bulk-sensitive-data-policy-beyond-doj-rule-requirements]
- 6. Research Ethics - StatPearls - NCBI Bookshelf - NIH [https://www.ncbi.nlm.nih.gov/books/NBK459281/]
- 7. Still Timely After All These Years: How the Belmont Report ... [https://acrpnet.org/2025/08/06/still-timely-after-all-these-years-how-the-belmont-report-continues-to-shape-research-ethics]
- 8. Basic Ethical Principles - Loyola Marymount University [https://academics.lmu.edu/irb/basicethicalprinciples/]
- 9. Ethical Principles - Inside NKU - Northern Kentucky University [https://inside.nku.edu/rgc/research-compliance/irb/resources/ethical-principles.html]
- 10. Guiding Ethical Principles - Research Support [https://researchsupport.psu.edu/orp/irb/information-for-participants/guiding-ethical-principles/]
- 11. Fostering Ethical Research in Contexts with Nuanced ... [https://www.tc.columbia.edu/institutional-review-board/irb-blog/2022/fostering-ethical-research-in-contexts-with-nuanced-vulnerability/]
- 12. Ethical Guidelines, Federal Regulations and State Statutes [https://rci.ucmerced.edu/irb/resources/ethical-guidelines-regulations-and-statutes]
- 13. Guiding Principles for Ethical Research [https://www.nih.gov/health-information/nih-clinical-research-trials-you/guiding-principles-ethical-research]
- 14. The Belmont Report - Ethical Principles [https://www.washington.edu/research/hsd/guidance/ethical-principles/belmont/]
- 15. Dual Use Research of Concern [https://www.k-state.edu/comply/committees/durc/]
- 16. Gold Standard Science at HHS [https://www.hhs.gov/gss/index.html]
- 17. The "Common Rule" | National Institute of Justice [https://nij.ojp.gov/funding/common-rule]
- 18. Justice in Clinical Studies: Guiding Principles - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK236544/]
- 19. FDA Draft Guidance on Multiregional Clinical Development ... [https://www.wcgclinical.com/insights/fda-draft-guidance-on-multiregional-clinical-development-programs-for-oncology/]
- 20. Utilizing tables, figures, charts and graphs to enhance the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10394528/]
- 21. 14 Comparing quantitative data between individuals [https://bookdown.org/pkaldunn/SRM-Textbook/BetweenQuantData.html]
- 22. 7 Types of Comparison Charts for Effective Data Visualization [https://ninjatables.com/types-of-comparison-charts/?srsltid=AfmBOorofcBkKDTbiJxLPE5O6KBkJopMIfiETmp3BMaq22c8Q0f4IBJq]
- 23. How the Belmont Report clarified informed consent [https://www.massdevice.com/how-the-belmont-report-clarified-informed-consent/]
- 24. An In-depth Look at Informed Consent | Resources [https://www.brandeis.edu/ora/hrpp/resources/in-depth-consent.html]
- 25. Research Ethics and Informed Consent [https://researchbasics.education.uconn.edu/ethics-and-informed-consent/]
- 26. Building trust in clinical research: a systems approach to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12307415/]
- 27. Informed Consent Guidelines & Templates [https://hrpp.umich.edu/irb-health-sciences-and-behavioral-sciences-hsbs/informed-consent-guidelines-templates/]
- 28. Vulnerability in research ethics: A systematic review of policy ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12212517/]
- 29. Patient priorities for fulfilling the principle of respect in research [https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-023-00954-5]
- 30. An Approach to a Benefit-Risk Framework [https://acrpnet.org/2024/04/12/an-approach-to-a-benefit-risk-framework]
- 31. Principles for Assessing Benefit and Harm in Systematic ... [https://www.ncbi.nlm.nih.gov/books/NBK115754/]
- 32. Methodological guidelines and publications of benefit–risk ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10729267/]
- 33. Conducting Risk-Benefit Assessments and Determining Level ... [https://ohrpp.research.ucla.edu/assessing-risks/]
- 34. 7 Types of Comparison Charts for Effective Data Visualization [https://ninjatables.com/types-of-comparison-charts/?srsltid=AfmBOorx7cLQFheTf83RIbUUWk7QEbQXP4JV1wJjxa6Xz4WPpEw0Ps74]
- 35. What to consider when choosing colors for data visualization [https://academy.datawrapper.de/article/140-what-to-consider-when-choosing-colors-for-data-visualization]
- 36. Your Data Visualization Color Guide: 7 Best Practices | Sigma [https://www.sigmacomputing.com/blog/7-best-practices-for-using-color-in-data-visualizations]
- 37. Qualitative vs. Quantitative Data in Research: The Difference [https://www.fullstory.com/blog/qualitative-vs-quantitative-data/]
- 38. Qualitative Vs. Quantitative Research – A Comparison [https://www.enago.com/academy/qualitative-vs-quantitative-research/]
- 39. Comparing Quantitative and Qualitative Research Methods [https://www.m3global.com/blog-comparing-quantitative-and-qualitative-research-methods-exploring-respondent-engagement.html]
- 40. Ethics and Equity: Addressing Violations of the Belmont ... [https://www.sciencedirect.com/science/article/pii/S2452292925000074]
- 41. Institutional Review Boards Frequently Asked Questions [https://www.fda.gov/regulatory-information/search-fda-guidance-documents/institutional-review-boards-frequently-asked-questions]
- 42. Institutional Review Boards: Purpose and Challenges - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4631034/]
- 43. IRB Background and Purpose [https://www.belmont.edu/irb/about/background.html]
- 44. The Three Principles Of The Belmont Report: Respect For ... [https://coursepivot.com/blog/which-of-the-following-are-the-three-principles-discussed-in-the-belmont-report/]
- 45. SPIRIT 2025 Statement: Defining standard protocol items ... [https://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/]
- 46. Constructing a finer-grained representation of clinical trial ... [https://www.nature.com/articles/s41597-023-02869-7]
- 47. Presenting data in tables and charts - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4008059/]
- 48. Healthcare Providers' Understanding of Data Displays ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10589436/]
- 49. Principles of Clinical Ethics and Their Application to Practice [https://pmc.ncbi.nlm.nih.gov/articles/PMC7923912/]
- 50. Clarification of ethical principle of the beneficence in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10061877/]
- 51. Ethical issues in palliative care: nursing and quality of life [https://bmcnurs.biomedcentral.com/articles/10.1186/s12912-024-02530-7]
- 52. Ethical conflict among critical care nurses during the COVID ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8795757/]
- 53. The Four Scientific Ethical Principles [https://researchethics.dk/research-ethics-themes/the-four-scientific-ethical-principles]
- 54. Ensuring ethical standards and procedures for research ... [https://www.who.int/activities/ensuring-ethical-standards-and-procedures-for-research-with-human-beings]
- 55. Ethics Challenges in Pediatric Research - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10715381/]
- 56. The Autonomy of Minors in the Doctor-Patient Relationship [https://healersandpatients.web.unc.edu/2024/04/the-autonomy-of-minors-in-the-doctor-patient-relationship/]
- 57. The Role of Parental Capacity for Medical Decision-Making ... [https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2020.559263/full]
- 58. General considerations for research with vulnerable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5151796/]
- 59. Optimizing Informed Consent-A Call to Action [https://pubmed.ncbi.nlm.nih.gov/40314939/]
- 60. Optimizing Informed Consent—A Call to Action [https://ge2p2global-centerforinformedconsentintegrity.org/2025/06/01/optimizing-informed-consent-a-call-to-action/]
- 61. Ways to Streamline Informed Consent Process in Clinical ... [https://www.advarra.com/blog/ways-to-streamline-informed-consent-process-in-clinical-trial-startup/]
- 62. Research ethics committees as knowledge gatekeepers [https://www.sciencedirect.com/science/article/pii/S2666659625000083]
- 63. Reframing data ethics in research methods education [https://educationaltechnologyjournal.springeropen.com/articles/10.1186/s41239-023-00380-y]
- 64. Consensus-based guidance for conducting and reporting ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8626083/]
- 65. - Genetic and Therapies | NHLBI, NIH Benefits Risks [https://www.nhlbi.nih.gov/health/genetic-therapies/benefits-risks]
- 66. Counseling Patients About Gene | Retinal Physician Therapy [https://www.retinalphysician.com/issues/2025/october/counseling-patients-about-gene-therapy/]
- 67. Quantitative risk analysis setup guide – master the process ... [https://www.trustcloud.ai/risk-management/master-quantitative-risk-analysis-a-step-by-step-guide-for-better-business-decisions/]
- 68. A Comprehensive Guide to Quantitative Risk Frameworks [https://www.metricstream.com/learn/quantitative-risk-frameworks.html]
- 69. Quantitative Risk Analysis: Its Importance and Implications [https://www.cisecurity.org/insights/blog/quantitative-risk-analysis-its-importance-and-implications]
- 70. Uncovering systemic barriers related to equity, diversity and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12359415/]
- 71. Institutional barriers to actionable science: Perspectives ... [https://www.sciencedirect.com/science/article/pii/S1462901121003592]
- 72. The most prevalent perceived barriers to sharing research ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12375187/]
- 73. A guideline for reporting experimental protocols in life sciences [https://pmc.ncbi.nlm.nih.gov/articles/PMC5978404/]
- 74. Chapter 15 Experimental protocols | Lab Handbook [https://ccmorey.github.io/labHandbook/experimental-protocols.html]
- 75. Text has enhanced contrast | ACT Rule | WAI [https://www.w3.org/WAI/standards-guidelines/act/rules/09o5cg/]
- 76. Implementation of Common Rule Changes to the Informed ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7122266/]
- 77. 45 CFR Part 46 -- Protection of Human Subjects [https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46]
- 78. Revised Common Rule - Institutional Review Board [https://www.belmont.edu/irb/common-rule.html]
- 79. The Next Phase of Human Gene-Therapy Oversight - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC6688383/]
- 80. Cellular & Gene Therapy Products [https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products]
- 81. Understanding FDA Cell and Gene Therapy Guidance [https://www.synthego.com/blog/fda-cell-and-gene-therapy-guidance/]
- 82. The Ethics of Human Genetic Intervention: A Postmodern ... [https://www.sciencedirect.com/science/article/pii/S0014488696964043]
- 83. Cellular & Gene Therapy Guidances [https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances]
- 84. RFI: Improving Americans' Access to Gene Therapies [https://www.asgct.org/advocacy/policy-statements/rfi-improving-americans-access-to-gene-therapies]
- 85. Nuremberg Code - UNC Research [https://research.unc.edu/human-research-ethics/resources/ccm3_019064/]
- 86. WMA Declaration of Helsinki – Ethical Principles for ... [https://www.wma.net/policies-post/wma-declaration-of-helsinki/]
- 87. Nuremberg Code [https://en.wikipedia.org/wiki/Nuremberg_Code]
- 88. Nuremberg Code | History, Date, & 10 Points [https://www.britannica.com/topic/Nuremberg-Code]
- 89. A Summary of Important Documents in the Field of Research ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2632196/]
- 90. Statement on the revision of the Declaration of Helsinki ... [https://www.ifpma.org/news/statement-on-the-revision-of-the-declaration-of-helsinki-on-ethical-principles-for-medical-research/]
- 91. Reports, Declarations, Codes & Guidelines - Academics [https://academics.lmu.edu/irb/reportsdeclarationscodesguidelines/]
- 92. The Declaration of Helsinki at 60: Bridging Historical Ethics ... [https://wfneurology.org/activities/news-events/archived-news/2025-02-25-Declaration-of-Helsinki-at-60]
- 93. Towards Principles-Based Approaches to Governance of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3885861/]
- 94. Proposing a Principle-Based Approach for Teaching AI Ethics ... [https://mededu.jmir.org/2024/1/e55368/]
- 95. The Role of Empirical Research in Bioethics - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC2826359/]
- 96. Researchers' Views on Doing Empirical Bioethics [https://link.springer.com/article/10.1007/s11673-023-10286-z]
- 97. Why 90% of clinical drug development fails and how to ... [https://www.sciencedirect.com/science/article/pii/S2211383522000521]
- 98. Acceptable objectives of empirical research in bioethics [https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-022-00845-1]
- 99. The History of the Belmont Report | 2020 | IRB Blog [https://www.tc.columbia.edu/institutional-review-board/irb-blog/2020/the-history-of-the-belmont-report/]
- 100. NIST Researchers Suggest Historical Precedent for Ethical ... [https://www.nist.gov/news-events/news/2024/02/nist-researchers-suggest-historical-precedent-ethical-ai-research]