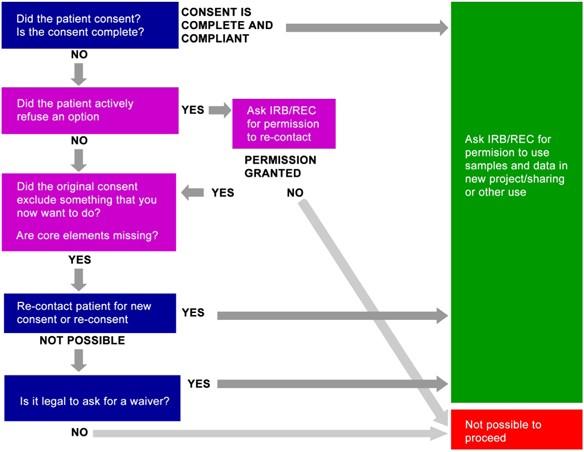
Research Articles
Assessing Patient Comprehension in Informed Consent: Strategies, Challenges, and Future Directions for Clinical Research
This comprehensive review addresses the critical challenge of patient comprehension in the informed consent process, a fundamental ethical requirement in clinical research and drug development.
Beyond the Form: Integrating Cultural Competence into the Informed Consent Process for Global Research
This article provides researchers, scientists, and drug development professionals with a comprehensive framework for implementing culturally competent informed consent processes.
Plain Language in Informed Consent: A Strategic Guide for Clinical Researchers
This article provides a comprehensive guide for researchers and drug development professionals on implementing plain language in informed consent forms (ICFs).
Emergency Research Informed Consent Waivers: A Comprehensive Guide to FDA Criteria, Implementation, and Ethical Compliance
This article provides a detailed examination of the Exception from Informed Consent (EFIC) requirements for emergency research, as defined by the FDA under 21 CFR 50.24.
Ethical Safeguards: A Comprehensive Guide to the Informed Consent Process for Vulnerable Populations in Clinical Research
This article provides researchers, scientists, and drug development professionals with a strategic framework for navigating the ethical and practical complexities of obtaining informed consent from vulnerable populations.
Common Rule Informed Consent: Complete Guide to Documentation Requirements for Researchers
This comprehensive guide details the informed consent documentation requirements under the revised Common Rule, essential for researchers, scientists, and drug development professionals.
A Comprehensive Guide to Obtaining Valid Informed Consent in Clinical Trials: Principles, Procedures, and Best Practices
This article provides a complete framework for researchers and clinical trial professionals to ethically and effectively obtain valid informed consent.
From Paternalism to Partnership: The Evolution of Patient Autonomy in Medical Ethics and Its Impact on Drug Development
This article provides a comprehensive analysis of the historical evolution, theoretical foundations, and practical applications of patient autonomy in medical ethics, with a specific focus on implications for researchers and...
From Patient Autonomy to Common Rule: The Definitive Timeline of Informed Consent Milestones
This article provides a comprehensive historical analysis of informed consent, tracing its evolution from early 20th-century legal foundations to modern regulatory frameworks.
Key Legal Cases That Established Informed Consent: A Guide for Research and Drug Development Professionals
This article provides a comprehensive analysis of the pivotal legal cases that established and shaped the doctrine of informed consent, from its early 20th-century foundations to recent court rulings.